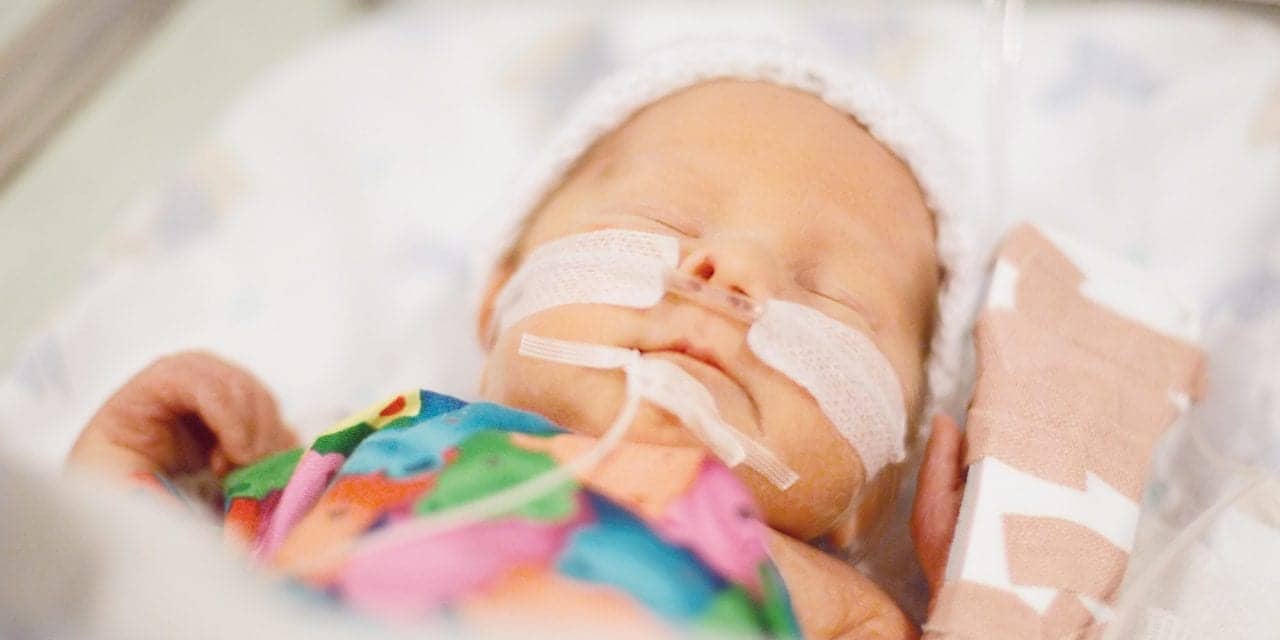
Persistent pulmonary hypertension of the newborn (PPHN) is a serious cardiopulmonary disorder characterized by elevated pulmonary vascular resistance that can lead to decreased pulmonary blood flow and hypoxic respiratory failure (HRF).1 The condition, which affects about 2 in every 1,000 live births, has a mortality rate of 7-10%, causes subsequent impairments in 25% of survivors due to cerebral hypoxia, and requires critical care interventions up to and including extracorporeal life support.2 In the last 25 years, however, the use of inhaled nitric oxide (iNO) in this population—FDA-approved since 1999—has demonstrated its effectiveness at improving oxygenation, reducing the need for ECLS (specifically ECMO), and saving PPHN neonates suffering HRF.3
In this edition of our “Industry Analysis” series, RT magazine speaks with manufacturers of inhaled nitric oxide delivery systems about the latest market innovations in the iNO industry, research on its potential use to treat other cardiopulmonary diseases, and what factors hospitals should weigh when considering an investment in the therapy.
Participating in the conversation were:
- Mark Rimkus, P.Eng, RRT, VP of Clinical Affairs, Beyond Air;
- Dana Saporito, MS, RRT-NPS, senior director of Medical Affairs at Mallinckrodt Pharmaceuticals; and
- Patricia-Ann Therriault, RRT, FCSRT, director of Medical and Clinical Affairs, Airgas Therapeutics.
RT: Use of inhaled nitric oxide therapy has been in practice in cardiorespiratory care for over 20 years. What is iNO currently indicated for and how does the therapy work?

Mark Rimkus, P.Eng, RRT (Beyond Air): In the United States, inhaled nitric oxide (iNO) is FDA-approved for the treatment of term and near-term neonates (≥34 weeks gestation) with hypoxic respiratory failure associated with clinical or echocardiographic evidence of pulmonary hypertension. It is indicated to improve oxygenation and reduce the need for extracorporeal membrane oxygenation (ECMO) when used alongside ventilatory support and other appropriate therapies.
Internationally, including regions like the European Union, Australia, and Japan, iNO is also indicated to selectively reduce pulmonary arterial pressure in neonates through adults with perioperative pulmonary hypertension associated with cardiac surgery.
Mechanistically, nitric oxide relaxes vascular smooth muscle by binding to the heme moiety of cytosolic guanylate cyclase, activating guanylate cyclase and increasing intracellular levels of cyclic guanosine 3′,5′- monophosphate, which then leads to vasodilation. When inhaled, nitric oxide selectively dilates the pulmonary vasculature, and because of efficient scavenging by hemoglobin, has minimal effect on the systemic vasculature. Nitric oxide appears to increase the partial pressure of arterial oxygen (PaO2) by dilating pulmonary vessels in better ventilated areas of the lung, redistributing pulmonary blood flow away from lung regions with low ventilation/perfusion (V/Q) ratios toward regions with normal ratios.

Dana Saporito, MS, RRT-NPS (Mallinckrodt Pharmaceuticals): Approved in December 1999 in the US, INOmax (nitric oxide) gas, for inhalation was the first selective pulmonary vasodilator indicated to improve oxygenation and reduce the need for extracorporeal membrane oxygenation in term and near-term (>34 weeks gestation) neonates with hypoxic respiratory failure associated with clinical or echocardiographic evidence of pulmonary hypertension in conjunction with ventilatory support and other appropriate agents.
INOmax works by opening up the blood vessels in the lungs to allow more blood and oxygen to flow through the body. Globally, INOmax is approved in Canada, Japan (approved as INOflo), and Australia. INOmax is also approved and distributed by partner companies in many countries such as Mexico and across, Europe, Africa, Asia, and South America. The approved indications differ depending on ountry or territory.
See Additional Notes: M1 (below) for Important Safety Information.

Patricia-Ann Therriault, RRT, FCSRT (Airgas Therapeutics): Ulspira Nitric oxide gas, for inhalation, is a vasodilator indicated to improve oxygenation and reduce the need for extracorporeal membrane oxygenation in term and near-term (>34 weeks gestation) neonates with hypoxic respiratory failure associated with clinical or echocardiographic evidence of pulmonary hypertension (PPHN) in conjunction with ventilatory support and other appropriate agents. Ulspira is contraindicated in neonates dependent on right-to-left shunting of blood. Prescribers should refer to ulspira.com for detailed indication and important safety information.
Inhaled nitric oxide is a selective pulmonary vasodilator which helps with perfusion of the lung and decreases the ventilation/perfusion ratio by ensuring adequate perfusion to well ventilated alveoli. It does this by acting on the nitric oxide pathway and cyclic guanosine monophosphate (GMP) which leads to smooth muscle relaxation. Because it is inhaled and selective to the pulmonary vasculature, and quickly binds to the red blood cell once it crosses the cell barrier into the blood, it does not cause systemic hypotension.
RT: Although FDA approval for the therapy is limited to PPHN, it is being researched as a potential treatment for additional disease states, including viral and bacterial infections, COVID-19-related pneumonia, and COPD, to name a few. What can you tell us about ongoing research into these diseases/disorders and what the data are revealing?
Rimkus: There is an exciting amount of active research studying the use of high-dose (150-250 ppm) intermittent iNO inhalations for treating viral pneumonia (including COVID-19 pneumonia), non-tuberculous mycobacterium infections, and viral bronchiolitis, and I am proud to say Beyond Air has been involved in much of it. There is still a significant amount of work to be done, but high dose iNO may be beneficial to patients suffering from these acute and chronic diseases.
Saporito: We continually look at ways to meet patient and customer needs while research for our products is ongoing. Detailed research information is available on clinicaltrials.gov.
RT: Tell us about your iNO delivery system. How it is innovative and what are some of the features and advantages it offers?

Rimkus: The LungFit PH system by Beyond Air is the first and only FDA-cleared inhaled nitric oxide delivery system that generates nitric oxide on-demand from ambient room air—completely eliminating the need for high-pressure cylinders or liquid chemicals.
Key innovations include:
- Proportional Delivery Across Wide Ranges: LungFit PH delivers proportional iNO across a broad range of flow rates (0.5–100 L/min) and dose settings (0–80 ppm). Its CMOS-based electronic flow sensor ensures rapid and accurate response times—under 30 seconds regardless of settings—and covers the entire range with a single disposable sensor.
- Simple, Safe Filtration: LungFit PH does not use any pressurized cylinders or liquid chemicals and only requires a small (60g/ 2.1oz) NO2 filter to remove the trace amount of NO2 generated alongside the NO. The filter is changed every 12 hours, regardless of set NO or ventilator settings. The changeout process takes less than 5 seconds, requires no special handling or storage, and the filters have a 2-year shelf life.
- Automated Safety Features: Because LungFit uses room air as a carrier gas, it automatically purges the injector line circuit during standby or pauses in treatment. This eliminates the risk of delivering NO2 boluses when resuming iNO delivery after a pause in therapy or a gap between device checkout and start of iNO therapy.
- Independent Continuous Gas Monitoring: The system continuously monitors NO, NO₂, and O₂ concentrations in real time. Importantly, the monitoring function is independent from the delivery system, meaning any issues with the gas sampling side—such as a blocked or leaking line—do not compromise the accuracy of iNO delivery.
- Redundant Ionization System: LungFit PH is equipped with a secondary NO ionizer, enabling immediate backup functionality should there ever be an issue with the primary delivery system. This feature also supports uninterrupted delivery during manual ventilation, allowing simultaneous delivery to both ventilator and manual resuscitation circuits. This seamless transition provides peace of mind during critical interventions.
Together, these innovations make LungFit PH a safer and more efficient alternative to traditional tank-based systems.

Saporito: The INOmax Evolve DS is a fully integrated delivery system that can be detached for intrahospital transport without the cart. Evolve also features improved automation that enhances safety features and streamlines the overall user experience, while still delivering on some of the core features from the INOmax DSIR Plus family such as dual-channel design and proportional INOmax delivery. Some of the innovative features are as follows:
- No high calibrations needed with factory-calibrated eINOcal Module. Low calibrations are automatic
- Multiple failover systems within the Evolve device activate automatically for continuation of INOmax delivery to the Injector Module, or through the eINOblender while setting up a replacement device
- Automatic cylinder switchover between the two integrated cylinder bays mitigates interruption of delivery when one cylinder is depleted
- Integrated eINOblender backup system automatically activates when a minimum O2 flow to the manual resuscitator is detected, and its dose is auto-set to match the primary delivery system dose
- EMR connectivity updates patient’s medical record with dose, monitored values, cylinder information including pressure, alarm information, and many other parameters
- Electronic injector module has bi-directional flow detection from -60 L/min reverse flow to 160 L/min forward flow. Low-flow accuracy is down to 0.5 L/min
- Touch-screen display has on-screen wizards that assist with automated pre-use checkout, alarm help, and on-screen ventilator setup
- Dedicated cylinder information screens show the cylinder percent full or runtime remaining
- Automated pre-use checkout procedure runs when initial connections are made
- Global status indicator, as well as primary delivery and eINOblender delivery port lights are also featured
See Additional Notes: M2 (below) for applications, device warnings, and additional info.
Therriault: Designed with precision and patient care in mind, the Ulspira Solution brings advanced therapy options to critical care environments, offering reliability, safety, and ease of use for healthcare providers.
An important point when initiating NO therapy is to be able to do so quickly, since patients are critically ill. Our Pre-Use Check and automatic purge of the system enables therapy to be started within a few minutes and prevents any NO2 boluses to the patient. Some key features also include a user-friendly and intuitive touchscreen, an automatic cylinder switch without drug interruption or the user having to manipulate the cylinders, as well as digital titration as low as 0.1 ppm increments which enables precise weaning in neonates. A gas therapy calculator is also available so the user can determine how long a tank will last under the current therapy.
Our consumables are single patient use and disposable, including our flow sensor and injection assembly as well as water trap and all other components that interface with the patient circuit. This ensures quick turn-around and adherence to infection control guidelines.
The Ulspira Care+ full product offer from Airgas Therapeutics includes the drug and device, clinical support and consumables and also Total Gas Management (TGM) service for all your cylinder and consumables logistics, ordering and transportation within the hospital.
The Ulspira TS device is FDA cleared and specifically marketed for the USA has been based on a platform called SoKINOX in Canada, Europe and Australia. It has been in the field for 8+ years and has undergone multiple software and hardware enhancements in order to improve usability, compatibility and reliability.
Ulspira TS must only be used in accordance with the indications, contraindications, warnings and precautions described in the nitric oxide drug packaging inserts and labeling, and is indicated for use in term and near-term (>34 weeks gestation) neonates with hypoxic respiratory failure associated with clinical or echocardiographic evidence of pulmonary hypertension. The primary targeted clinical setting is the Neonatal Intensive Care Unit (NICU) and secondary targeted clinical setting is the transport of neonates. Refer to ulspira.com for Ulspira TS Important Safety Information.
RT: Will delivery system advancements and integrated monitoring solutions ever allow iNO to be administered in additional clinical settings, such as the home?
Rimkus: There is potential for iNO delivery systems to expand into non-ICU settings as devices become simpler to operate and manage. Improvements in emergency backup functionality, elimination of large gas cylinders, and user-friendly operation could make iNO more feasible for intermediate care units or transport environments.
However, home use remains a complex challenge. iNO is short-acting and requires precise dosing and monitoring, as abrupt interruption of iNO delivery could lead to rebound pulmonary hypertension. This makes continuous home therapy less practical compared to long-acting nebulized vasodilators, such as prostacyclin analogues, which are more suitable for chronic outpatient management.
That said, intermittent high-dose iNO therapy (>150 ppm) is an exciting frontier for potential at-home use. Beyond Air recently supported a 12-week pilot study in Australia involving patients with persistent non-tuberculous mycobacterium (NTM). Patients self-administered 250 ppm iNO treatments twice daily at home for 40 minutes per session. The investigational system demonstrated both safety and feasibility in the home environment, paving the way for further research and development.
Saporito: As patient care continues to advance, we look at ways to be able to support their needs. We will evaluate other uses in clinical settings for future considerations.
RT: What factors should hospitals consider when choosing an iNO delivery system? And for hospitals considering investment in the therapy, what is your advice for how to balance the capital costs, staff training, and other financial impacts with the clinical benefits for patients?
Rimkus: As a respiratory therapist, I see the ideal iNO delivery system as one that delivers accurately while simultaneously minimizing the time and attention required from the clinical team. Think of it like an IV pump: I want to set it up quickly, input the dose, and trust it to operate reliably with minimal interaction. That way, we can stay focused on patient care—not device management.
Hospitals should look for systems that are intuitive, quick to set up, and easy to maintain. Routine interactions like filter or cartridge changes should be clearly prompted, predictable, and simple. When a system reduces complexity and burden, training becomes easier, and staff are more confident using it.
Financially, hospitals should consider not just capital cost but the total cost of ownership—including consumables, downtime, staffing demands, storage, and waste disposal. By eliminating cylinders, LungFit PH simplifies logistics, reduces hidden costs, and supports hospitals in meeting their clinical, operational, and environmental goals.
Saporito: When selecting an iNO delivery system, it is critical to prioritize clinical effectiveness and safety, especially for the most fragile and acutely ill patients. While acquisition cost and operating costs are important, so is choosing a delivery system equipped with features guided by input from respiratory therapists and built on a decades-long foundation of partnership.
A critical aspect of iNO delivery systems is their backup system which ensures uninterrupted iNO delivery in case of device malfunction. Backup systems should be intuitive and capable of automatically activating when required. Back-up systems that are fully integrated offer enhanced portability for our users.
An important feature for an INO delivery system is ‘dual channel’ configurations. Though single-channel delivery systems integrate delivery and monitoring within the same pathway such that the monitoring feeds information back to the delivery controller, that is then verified by the monitor, completing a feedback loop, the dual-channel delivery systems have separate, independent delivery and monitoring systems, allowing each system to function independently. The dual channel can help mitigate the risk of delivery interruption and the risk of incorrect dosing not due to operator error.
Another factor for consideration is whether the devices used in a hospital have been validated with the iNO delivery system. ‘Validation’ confirms that the iNO device has been tested by an expert, meets specified requirements and performs as intended. The validation process involves standardized, rigorous testing methodology and reporting results to the FDA which in turn results in a 510K clearance for the devices to be utilized simultaneously. Though ’compatibility’ suggests that the iNO device can function with other medical equipment it does not mean that the two devices have been validated or tested by an expert or reviewed by the FDA.
Hospitals should also consider the availability of access for emergencies. The INOmax Total Care (ITC) offering provides 24/7/365 US-based service along with our RT-Based Product Support and a Clinical Specialist Team to assist with device-related questions when a need arises. We have regional service centers across the country to facilitate emergency equipment response if necessary. Emergency deliveries are most often made within 4 to 6 hours but can take up to 24 hours depending on the hospital location and/or circumstance. The ITC offering has a proven track record for the last 25 years, including 98.6% of on-time deliveries in 2024.
Therriault: Multiple factors go into choosing a nitric oxide provider. Some of these include the company’s size and service profile, reputation, end to end logistics and supply chain control, reliability, safety and usability of their device, and experience of clinical staff supporting the product 24/7.
Flexibility on invoicing models to match hospital’s needs is key. The Ulspira offer allows hospitals to choose between different business models like: cost per hour of use, flat fees, time packages, cost per treatment or per patient, device rental and cost per cylinder used.
It’s important to choose a provider that offers multiple options and a customer-centric approach.
RT: What other advancements in technology, or expanded clinical indications, do you foresee in the next five years and beyond?
Rimkus: There is a lot of interesting research exploring the benefits of adding NO to ECMO sweep gas (sNO). The immuno-modulatory and decreased platelet aggregation effects of NO may have a great impact on extending circuit life and decreasing plasma free hemoglobin generation, as well as the potential to decrease neurologic injury. There is much to be proven and understood about this, but it looks very promising. Technically, the ability to deliver accurately at low sweep gas flow rates will be important for smaller patients, and an easy-to-use delivery device will also prove valuable when it is added to the long list of duties (ECMO system, CRRT, anti-coagulation, fluid and blood replacement, etc) an ECMO specialist needs to manage during a shift.
Indications using high-dose (>150 ppm) iNO will be an exciting area to watch as well, but these are in quite early stages of development. These early studies are exploring its use for viral pneumonia, bronchiolitis, non-tuberculous mycobacterium and other bacterial infections associated with Bronchiectasis and Cystic Fibrosis, as well COPD exacerbation management. While all exciting potential areas of focus, much work needs to be done in these areas to determine the best dose and treatment schedule to maximize benefits.
Saporito: Similar to when we launched the INOmax DS, The INOmax Evolve DS is a platform we plan to regularly upgrade with new features and improvements. The INOmax Evolve DS is also designed to be capable of supporting new indications. At this time, due to regulatory reasons, we cannot comment further on the details or timing of upgrades or new indications that are in development.
Therriault: Future technical advancements should prioritize enhanced safety protocols, intuitive user interfaces, and streamlined workflows to minimize use errors. Technological strides will likely yield smaller, lighter devices capable of delivering increasingly precise doses, with automation handling routine tasks.
Expanding clinical indications for iNO therapy presents challenges due to the generic nature of the drug, which limits companies’ willingness to invest in extensive clinical trials. The current market, with approximately 30% off-label usage and standard of care practices, further complicates the pursuit of additional indications, unless a device or drug targets a highly specific application. While some European countries have expanded approvals the North American regulatory landscape remains more intricate. Ultimately, iNO, like any medication, falls under physician discretion, who determines its appropriate application based on individual patient needs.
| ADDITIONAL NOTES | |
|---|---|
| M1 | Important Safety Information: INOmax is contraindicated in the treatment of neonates dependent on right-to-left shunting of blood. Abrupt discontinuation of INOmax may lead to increasing pulmonary artery pressure and worsening oxygenation. Methemoglobinemia and NO2 levels are dose dependent. Nitric oxide donor compounds may have an additive effect with INOmax on the risk of developing methemoglobinemia. Nitrogen dioxide may cause airway inflammation and damage to lung tissues. In patients with pre-existing left ventricular dysfunction, INOmax may increase pulmonary capillary wedge pressure leading to pulmonary edema. Monitor for PaO2, inspired NO2, and methemoglobin during INOmax administration. INOmax must be administered using a calibrated FDA-cleared Nitric Oxide Delivery System. Additional Information can be found in the Prescribing Information. [pdf] https://www.inomax.com/downloads/Inomax-PI.pdf |
| M2 | Applications The INOmax Evolve DS and DSIR Plus delivery systems deliver INOmax (nitric oxide) gas, for inhalation. These delivery systems must only be used in accordance with the indications, usage, contraindications, and warnings and precautions described in the INOmax package insert and labeling and are indicated for use in term and near term (>34 weeks gestation) neonates with HRF associated with clinical or echocardiographic evidence of pulmonary hypertension. These delivery systems are indicated for a maximum of 14 days of use. Device Warnings Abrupt discontinuation of INOmax can lead to worsening oxygenation and increasing pulmonary artery pressure (rebound pulmonary hypertension syndrome). To avoid abrupt discontinuation, use the Evolve DS eINOblender or the DSIR Plus INOblender as a backup immediately to reinstate INOmax therapy and refer to the INOmax package insert. Do not discontinue INOmax delivery if the high NO2 alarm activates. Assess the delivery system for proper setup while maintaining INOmax delivery and verify INOmax and/or FiO2 are appropriate. Do not use equipment that is not specified as part of the systems or that is not designed for INOmax mixtures. Using equipment that is not specified can cause the systems to malfunction. If an alarm occurs, safeguard the patient first before performing troubleshooting procedures. Use only INOmax, pharmaceutical grade NO. Additional information can be found in the Operator’s Manual. |
Information provided by Mallinckrodt Pharmaceuticals.
RT
The responses above were provided by manufacturer representatives and are published here in their full, unedited form. RT strives for accuracy in all data but cannot be held responsible for claims made by marketers. Please refer to all product safety information. For more information, contact [email protected].
References
- Nandula PS, Shah SD. Persistent Pulmonary Hypertension of the Newborn. StatPearls Publishing; updated 2023 Jul 31. https://www.ncbi.nlm.nih.gov/books/NBK585100/
- Persistent Pulmonary Hypertension in the Neonate (PPHN). The Cleveland Clinic. https://my.clevelandclinic.org/health/diseases/16020-persistent-pulmonary-hypertension-in-the-neonate-pphn
- Sokol GM, et al. Inhaled nitric oxide therapy for pulmonary disorders of the term and preterm infant. Semin Perinatol. 2016 Oct;40(6):356-369. https://pmc.ncbi.nlm.nih.gov/articles/PMC5065760.