Federal Home Testing Program Expands Nationwide, Adding Flu Services
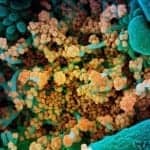
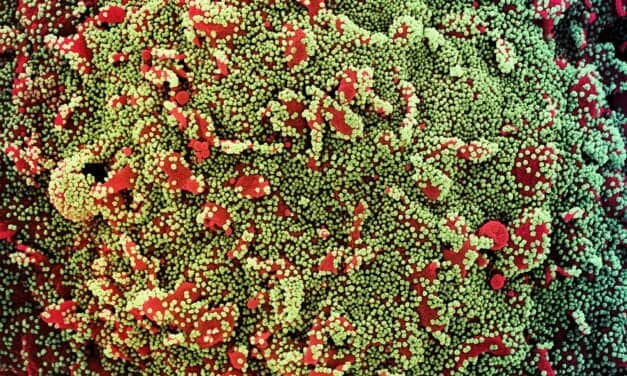
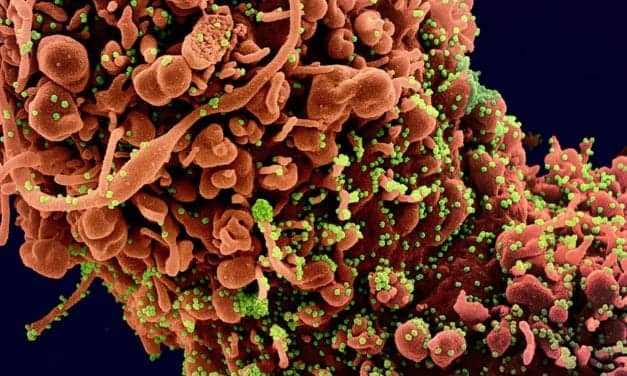
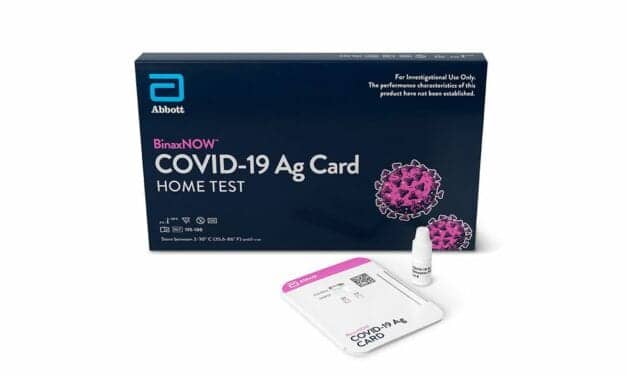
The federal government has expanded the Home Test to Treat program, a virtual health program offering free COVID-19—and now influenza—health services to eligible participants nationwide.