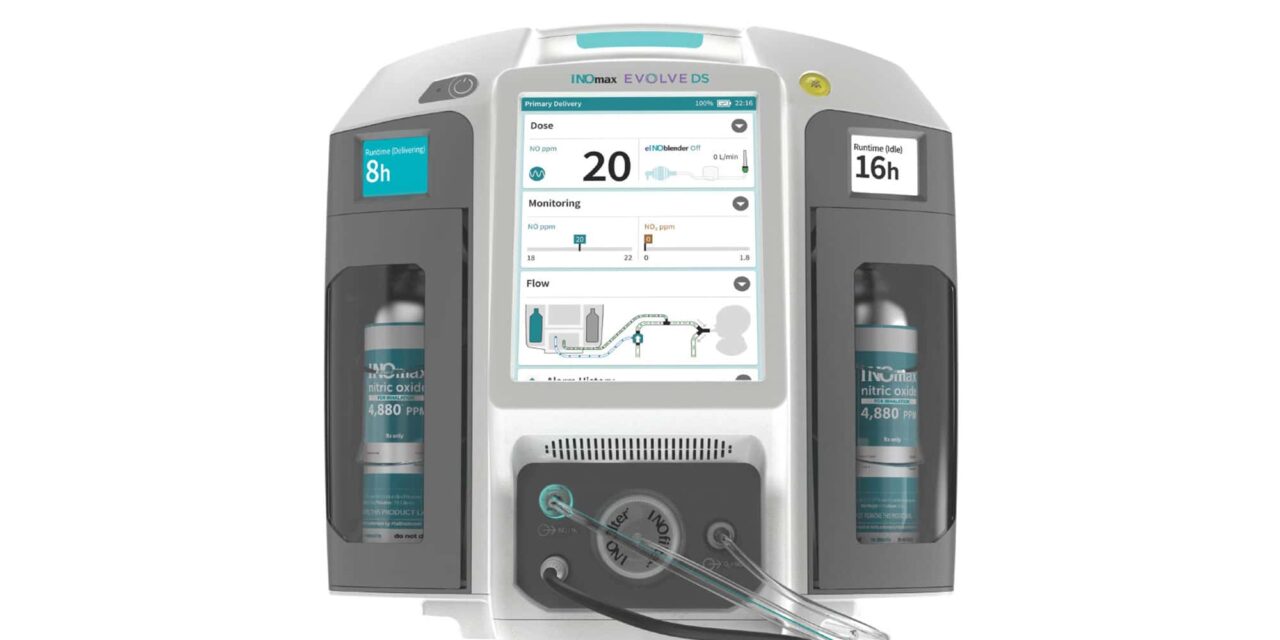
The FDA has cleared the INOmax Evolve DS for the delivery of INOmax nitric oxide gas for inhalation, according to Mallinckrodt plc.
The INOmax Evolve DS is the company’s next-generation nitric oxide delivery system with a fully integrated design that includes a primary delivery system, a monitoring system, an electronic blender, automated backup delivery, mini-cylinders, and more.1
According to Mallinckrodt, the device can help meet the needs of NICU patients and providers by offering improved automation, which enhances safety features, portability for intrahospital transport, and a streamlined design that elevates the user experience.1
“This achievement reflects our commitment over the past two decades to serve the needs of NICU patients by providing a next-generation INOmax delivery system with comprehensive safety features,“1 said Peter C. Richardson, MRCP (UK), Mallinckrodt executive VP and chief scientific officer. “The FDA clearance of the INOmax Evolve DS is a major milestone for our company, the dedicated team members who made this possible, and most importantly, for our critically ill patients and the NICU staff responsible for their care.”
INOmax Evolve DS delivers INOmax nitric oxide into the inspiratory limb of the patient breathing circuit in a way that provides a constant concentration of NO, as set by the user, to the patient throughout the inspired breath.
INOmax is an FDA-approved treatment that is indicated to improve oxygenation and reduce the need for extracorporeal membrane oxygenation in term and near-term (>34 weeks gestation) neonates with hypoxic respiratory failure associated with clinical or echocardiographic evidence of pulmonary hypertension in conjunction with ventilatory support and other appropriate agents.2
The device uses a specially designed injector module, which enables tracking of the gas delivery system waveforms and the delivery of a synchronized and proportional dose of NO. It may be used with the ventilators and respiratory care devices for which INOmax Evolve DS has been validated.1
The INOmax Evolve DS is not currently available for purchase, distribution, or use, but is expected to be available in US hospitals in the first half of 2024, according to the company.
References
1 Data on File – Ref-05943. Mallinckrodt Pharmaceuticals.
2 INOmax. Package insert. INO Therapeutics LLC; 2023.
3 Data on File – Ref-01753. Mallinckrodt Pharmaceuticals.