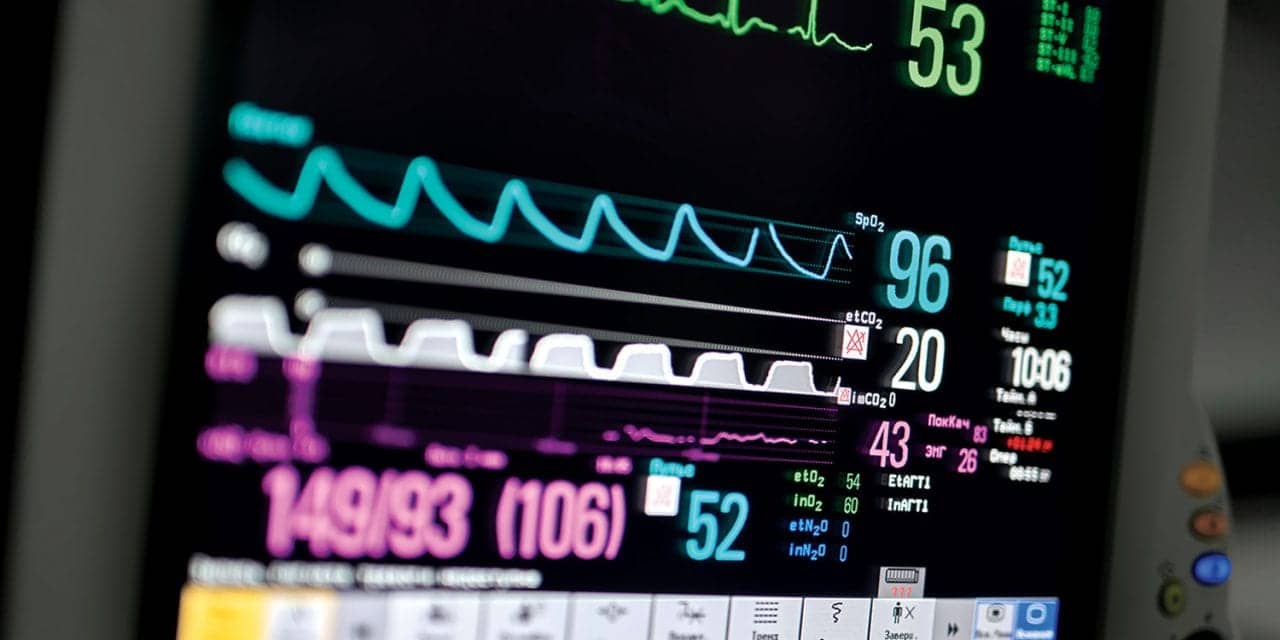
For patients in the pediatric ICU, pulse oximetry and waveform capnography are valuable tools to monitor the effectiveness of respiratory support and to inform treatment decisions.
By Bill Pruitt, MBA, RRT, CPFT, FAARC
For patients in the pediatric intensive care unit (PICU), pulse oximetry and waveform capnography are valuable tools to monitor the effectiveness of respiratory support and to inform treatment decisions. The gold standard for monitoring oxygenation and ventilation is the measurement of arterial blood gases (ABGs)—specifically, by examining PaO2, SaO2 for oxygen levels and PaCO2 for adequacy of ventilation. However, ABGs are costly, require some minutes of time to access data, provide only a snapshot of the parameters at the time of the sampling, require an arterial catheter or arterial puncture (which causes pain and discomfort), and may introduce problems such as infection, bleeding, loss of blood volume in the smaller patients.1
In contrast, pulse oximetry and capnography can provide continuous monitoring by noninvasive means and the technology is improving to make these approaches even more accurate and more useful. This article will explore the current use of these two monitors in the PICU, challenges in monitoring pediatric patients, and discuss available technology.

PICU Pulse Oximetry
Early technology in pulse oximetry relied on using two wavelengths of red and infrared light to measure the levels of oxyhemoglobin versus deoxyhemoglobin and provide an estimation of the hemoglobin’s O2 saturation along with pulsatile blood flow to measure heart rate. The SpO2 has acceptable correlation with the ABG’s SaO2 but pulse oximetry has issues with accuracy in the face of dyshemoglobins, melanin, bilirubin, and nail polish.1 Studies looking at pulse oximeter results for SpO2 versus ABG results for SaO2 have found that occult (hidden) hypoxemia is an issue when considering race/ethnicity. Asian, Black, and non-Black Hispanic patients, compared to white patients, had occult hypoxemia (generally defined as a SaO2 of <88% compared to a SpO2 of >92%) at twice to three times the rate.2-4 Moreover, it appears that this was linked to administration of less supplemental oxygen in the Asian, Black, and non-Black Hispanic patients compared to white patients.2 Research in this area has found that errors in pulse oximeter readings are caused by skin color differences and discrepancies are higher in patients with darker skin.4
Researchers have usually focused on adults as they explored this problem, but racial bias in children has also been documented.5 A project published in a research letter from 2023 in JAMA Pediatrics compared SaO2 to SpO2 in 774 children undergoing cardiac catheterization during 2016 to 2021 at The Children’s Hospital of Philadelphia. The most common diagnoses uncovered during the cardiac catheterization included hypoplastic left heart syndrome/single ventricle, tetralogy of Fallot, and patent ductus arteriosus. In the patients with true hypoxemia (SaO2 < 88%) the SpO2 had false negative readings (reflected in normal oxygen saturation or SpO2 >92%) in 12% of Black or African American patients versus 4% of white patients. On the other hand, in patients with normal pulse oximeter readings (SpO2 > 92%), 5% of Black or African American patients had true hypoxemia (SaO2 < 88%) versus 1% of white patients.5
Other confounding factors affecting accuracy included poor perfusion, tissue edema, patient movement (motion artifact) and ambient light. The technology has improved greatly and now devices may have up to 7 measured light wavelengths to provide a more accurate SpO2 measurement. This has allowed for providing an estimate of methemoglobin, carbon monoxide saturation, and total hemoglobin concentration. In addition, there is less interference with ambient light and reduces issues with motion artifact.1
However, there appears to still be an issue with race/ethnicity discrepancies and higher SpO2 readings compared to the SaO2. The Food and Drug Administration (FDA) has responded to this issue and changed the minimum mean accuracy of pulse oximeters. The SpO2 readings must be compared directly to SaO2 over the range of 70 to 100%. The pulse oximeters are considered accurate if 66% of the SpO2 values are within 2-3% of the SaO2 values and some 95% of the SpO2 values are within 4-6% of the SaO2.4 There are new approaches being evaluated to try and address the racial bias in pulse oximetry, including photoacoustic imaging and polarized light oximetry. When light is absorbed by the skin it generates sound waves. Changes in skin tone alters the soundwaves and researchers are using photoacoustic imaging to try and improve the accuracy of pulse oximeters with this approach. Polarized light oximetry uses polarization to reduce the effects of melanin on reading oxygen saturation.6
Researchers have been looking at pulse oximetry results to predict the success of oxygen therapy using high-flow nasal cannula devices. One strategy involves determining the Respiratory Rate-Oxygenation Index (ROX index), defined as: (SpO2/FiO2)/respiratory rate.
In a meta-analysis published in 2022, the authors concluded that, “our meta-analysis reveals that ROX index is an easy-to-use and promising tool for clinicians to identify patients with a higher risk of HFNC failure, and those with a lower value of ROX index must be carefully monitored, accompanied by dynamic evaluation of intubation indications. Additional studies are required to determine the best cut-off value and the proper acquisition time of ROX index in the future.”7
PICU Capnography
Capnography measures the partial pressure of exhaled carbon dioxide in relationship to time, or in relationship to exhaled volume with the display showing the value for the end tidal CO2 (PetCO2). Many devices include a display of the waveform. (See Graphic 1.)
Capnography is valuable in many applications, including detecting correct placement of an endotracheal tube (or loss of the airway), detecting apnea, the return of spontaneous circulation during cardiopulmonary resuscitation (CPR), adequacy of ventilation, monitoring during procedural sedation, polysomnography, patient transport, etc.8
Figure 1. Waveform Capnography
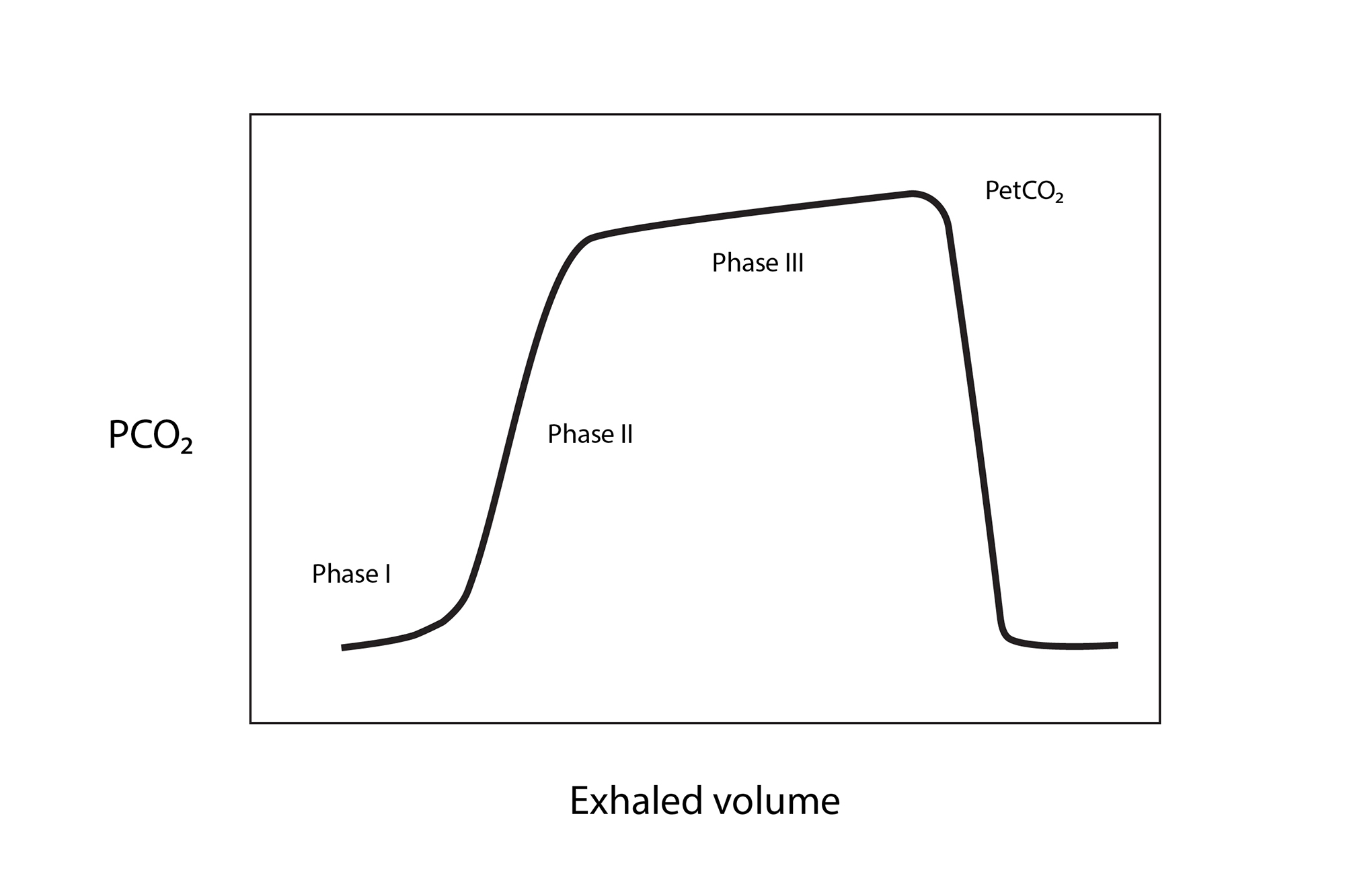
Capnography is performed using an infrared sensor to detect CO2 in the exhaled gas or using a pH sensitive material that changes color (colorimetric) in the presence of CO2. The infrared sensor can be either placed in the exhaled gas stream (mainstream) or use a sampling tube to pull a small portion of the exhaled gas into the sensor (sidestream). Mainstream capnography is used in intubated patients while sidestream monitoring can be use in both intubated and non-intubated patients. In nonintubated patients a nasal cannula is often used to provide the sample, and some cannulas have the capacity to provide supplemental oxygen through one side of the nasal prongs while obtaining the exhaled CO2 sample from the other side, using separate channels to connect to an oxygen source and a CO2 monitor. Other cannula designs have the usual two prongs for the nares which can provide supplemental oxygen plus a CO2 detection port that hangs down in front of the mouth.9 End-tidal CO2 cannulas are available in different sizes to accommodate neonates through adults, and many include a water-trap to help avoid issues with blocked flow due to moisture.
Colorimetric sensors are disposable, single-use devices and are used when intubating. With the colorimetric device placed between the end of the endotracheal (ET) tube and a resuscitator bag, correct ET tube placement is verified by the changing color in the device’s window—indicating the presence of CO2 (while an esophageal placement would not show any color change).8 Use of a colorimetric device at the end of the ET tube introduces dead space and rebreathing, so manufacturers offer a smaller device for use with children compared to the adult version of the device. It should be noted that colorimetric monitoring for ET tube placement can be less reliable if there is no cardiac output or if pulmonary blood flow is absent. In these situations, the device will not show the presence of CO2 and a correctly-placed ET tube may be mistakenly thought to be in the esophagus.8 Regardless, the use of colorimetric monitoring during intubation has great value in verifying correct ET tube placement and has become a universal standard of care, reflected in several organizations and professional bodies in the standards of practice, clinical practice guidelines, and practice recommendations.1,8
PetCO2 is usually 2-5 mmHg lower than the PaCO2 from an arterial blood gas in healthy patients. In diseases that alter the ventilation/perfusion (V/Q) ratio, the difference between the PetCO2 and the PaCO2 will increase. Examples of diseases that increase the Pa-etCO2 difference include ARDS, COPD, and asthma. Increase in physiologic deadspace, shunt, or low cardiac output are the main underlying causes of the widening Pa-etCO2 difference.
When patient-controlled analgesia (PCA) pumps are used, several organizations have recommended the use of PetCO2 monitoring to avoid over-sedation and respiratory depression.10 During CPR the effectiveness of chest compressions can be assessed, with the recommendation that the PetCO2 target threshold should be >10 mmHg. With continuous monitoring during CPR, a sudden increase in the PetCO2 is seen as the return of spontaneous circulation.1,8
Volumetric capnography (plotting PetCO2 over exhaled volume) has allowed clinicians to measure the dead space to tidal volume ratio (VD/VT). Knowing the VD/VT has been shown to help measure the severity of lung injury from ARDS and predict the risk of death. This measurement has also proven valuable in monitoring lung recruitment and avoiding overdistension in ARDS, and may be useful in predicting successful extubation in adult and pediatric patients. Finally, VD/VT can help in assessing the presence and severity of pulmonary embolism.8
Monitoring nonintubated children in PICU for adequacy of ventilation is a challenge. The subjective means of checking ventilatory status includes chest expansion, respiratory rate and depth of breathing, use of accessory muscles, and auscultation. However, these observations are variable from one evaluator to the next, involve intermittent checks on status, and impending respiratory distress/failure may be delayed or missed altogether.
While pulse oximetry can provide a continuous monitor for oxygenation, it does not monitor adequacy of ventilation and often will not uncover hypoventilation.9 Use of capnography has been expanding in monitoring children—starting with polysomnography, during procedural sedation, and in patient transport between facilities—to more widespread use of bedside monitoring. End-tidal CO2 monitoring has been shown to be an effective monitor at the bedside in PICU even in the face of high respiratory rates and low tidal volumes in this patient population.9
Conclusion
Monitoring patients in PICU is challenging but effective monitoring improves safety, helps guide care management, and can detect issues early-on to allow for preventive action to correct potential problems. Pulse oximetry has become a standard of care across all ages and in all care settings. Advances in pulse oximeter technology have allowed for monitoring many items beyond hemoglobin oxygen saturation and heart rate. However, there is a problem with different skin tones and it is negatively affecting the delivery of care in patients with darker skin. Research is going forward to try and reduce or eliminate this issue.
Capnography is also beginning to be a standard of care in PICU as it has many uses in monitoring and detecting changes in ventilation, verifying intubation, helping guide noninvasive and invasive ventilation, etc. These two approaches to monitoring can provide better, safer care and alert the healthcare team to potential detrimental changes in oxygenation and ventilation, and help reduce the number of arterial blood gases—thus reducing cost, risk of infection, pain, blood loss, etc. It is important for respiratory therapists to understand the changing technology, the effects of different physiological conditions on the readings, and the advantages and disadvantages/limitations of these two monitors.
RT
Bill Pruitt, MBA, RRT, CPFT, FAARC, is a writer, lecturer, and consultant. Bill has over 40 years of experience in respiratory care in a wide variety of settings and has over 20 years teaching at the University of South Alabama in Cardiorespiratory Care. Now retired from teaching, Bill continues to provide guest lectures, participates in podcasts, and writes professionally. For more info, contact [email protected].
References
- Schmidt GA. Monitoring gas exchange. Respiratory care. 2020 Jun 1;65(6):729-38.
- Gottlieb ER, Ziegler J, Morley K, Rush B, Celi LA. Assessment of racial and ethnic differences in oxygen supplementation among patients in the intensive care unit. JAMA internal medicine. 2022 Aug 1;182(8):849-58.
- Fawzy A, Wu TD, Wang K, Robinson ML, Farha J, et. al. Racial and ethnic discrepancy in pulse oximetry and delayed identification of treatment eligibility among patients with COVID-19. JAMA internal medicine. 2022 Jul 1;182(7):730-8.
- Abe T, Takagi T, Fujii T. Update on the management of acute respiratory failure using non-invasive ventilation and pulse oximetry. Annual Update in Intensive Care and Emergency Medicine 2023. 2023 Mar 25:165-75.
- Ruppel H, Makeneni S, Faerber JA, Lane-Fall MB, Foglia EE, O’Byrne ML, Bonafide CP. Evaluating the accuracy of pulse oximetry in children according to race. JAMA pediatrics. 2023 May 1;177(5):540-3.
- Gray KD, Subramaniam HL, Huang ES. Effects of Racial Bias in Pulse Oximetry on Children and How to Address Algorithmic Bias in Clinical Medicine. JAMA pediatrics. 2023 May 1;177(5):459-60.
- Junhai Z, Jing Y, Beibei C, Li L. The value of ROX index in predicting the outcome of high flow nasal cannula: a systematic review and meta-analysis. Respiratory Research. 2022 Dec;23(1):1-0.
- Siobal MS. Monitoring exhaled carbon dioxide. Respiratory care. 2016 Oct 1;61(10):1397-416.
- Coates BM, Chaize R, Goodman DM, Rozenfeld RA. Performance of capnometry in non-intubated infants in the pediatric intensive care unit. BMC pediatrics. 2014 Dec;14:1-6.