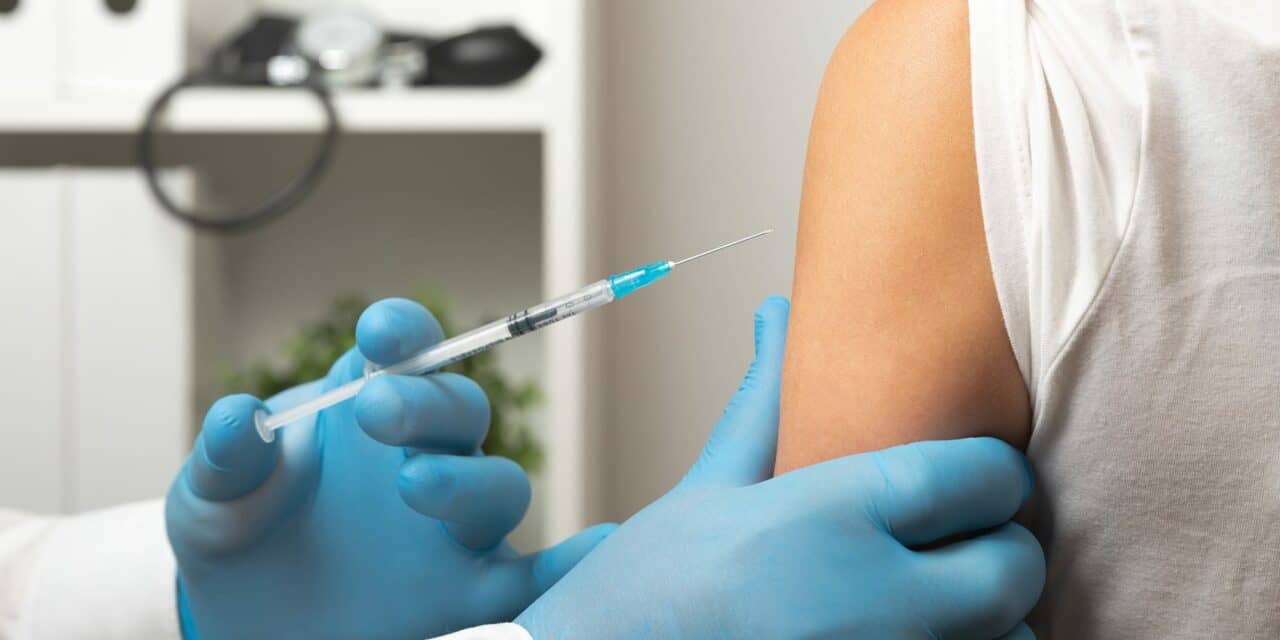
The US FDA has expanded the approval for Merck’s Vaxneuvance for prevention of invasive disease caused by Streptococcus pneumoniae to include children 6 weeks through 17 years of age. Vaxneuvance had been previously approved for adults 18 and older in July 2021.
The expanded indication means Vaxneuvance is the first pneumococcal conjugate vaccine approved in almost a decade to help protect pediatric populations against invasive pneumococcal disease, according to the company.
Vaxneuvance Pneumococcal 15-valent Conjugate Vaccine (pronounced VAKS-noo-vans) is now indicated for active immunization for the prevention of invasive disease caused by Streptococcus pneumoniae serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F and 33F in individuals 6 weeks of age and older.
Vaxneuvance is for intramuscular injection only and each dose is 0.5 mL. The vaccines is administered as a four-dose series at 2, 4, 6, and 12 through 15 months of age and administered as a single dose in children and adolescents 2 through 17 years of age who have received an incomplete series of another pneumococcal conjugate vaccine.
“Despite decreases in incidence of invasive pneumococcal disease in children, certain key serotypes continue to cause serious illness that can lead to death in children under the age of 5, with serotypes 3, 22F and 33F responsible for more than a quarter of all invasive pneumococcal disease cases in this population,” said Dr. Steven Shapiro, chairman, department of pediatrics, Jefferson Abington Hospital, and investigator for the PNEU-PED trial. “With the robust clinical data supporting Vaxneuvance and this FDA approval, Vaxneuvance will be an important new option to help advance protection for children.”
Invasive pneumococcal disease (IPD) is an infection caused by the bacterium Streptococcus pneumoniae, or pneumococcus. While there are approximately 100 different types of S. pneumoniae, called serotypes, a smaller number of serotypes are responsible for IPD in children. Serotypes 3, 22F and 33F are three of the top five serotypes causing childhood cases of IPD. IPD can lead to hospitalization or death. Some examples of IPD are bacteremia (an infection in the blood) and meningitis (an infection of the coverings of the brain and spinal cord), which can also result in long-term neurological complications. Children under the age of 2 are particularly vulnerable to IPD.
The FDA’s approval was based on data from seven randomized, double-blind clinical studies assessing safety, tolerability and immunogenicity of Vaxneuvance in infants, children and adolescents (see “Clinical Data Supporting FDA Approval” below for additional details). Clinical data from the pivotal study showed that immune responses elicited by Vaxneuvance following a four-dose pediatric series were non-inferior to the currently available 13-valent pneumococcal conjugate vaccine (PCV13) for the 13 shared serotypes based on serotype-specific immunoglobulin G (IgG) geometric mean concentrations (GMCs).
In a secondary analysis, immune responses for Vaxneuvance following a four-dose pediatric series were superior to PCV13 for shared serotype 3 and the two serotypes unique to Vaxneuvance, 22F and 33F. Randomized controlled trials assessing the clinical efficacy of Vaxneuvance compared to PCV13 have not been conducted.
Data from the clinical program also support the use of Vaxneuvance concomitantly with other commonly administered routine pediatric vaccines, and in a variety of clinical settings, such as interchangeable use following initiation of an infant vaccination schedule with PCV13 or in a catch-up setting for older children who are either pneumococcal vaccine-naïve or who previously received an incomplete series of another PCV. Additionally, data support the use of Vaxneuvance in special populations, such as in preterm infants and children living with HIV infection or sickle cell disease.
“Our goal with Vaxneuvance is to expand coverage of key invasive disease-causing serotypes and provide a strong immune response to serotypes that pose substantial risk to infants and children,” said Dr. Eliav Barr, senior vice president, head of global clinical development and chief medical officer, Merck Research Laboratories. “With this approval, we bring forward our first pediatric pneumococcal conjugate vaccine – and the first pediatric pneumococcal conjugate vaccine to be approved in almost a decade – building on our commitment to preventing invasive pneumococcal disease and on our legacy in pediatric vaccine development. We thank the investigators and the families of our clinical trial participants for participating in the research studies and the role they played in this milestone.”
More information including safety indications is available at Merck’s website.