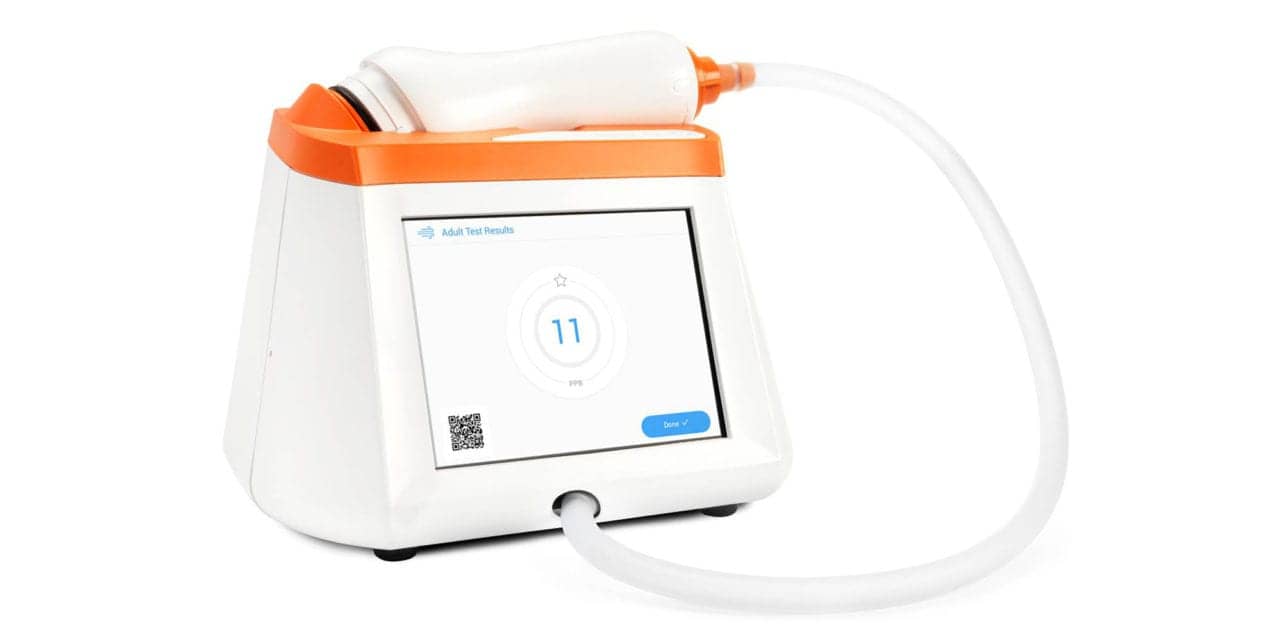
Caire’s Fenom Pro fractional exhaled nitric oxide (FeNO) device can now be upgraded to include a 6-second test mode available for ages 6 and up, according to a company news release.
The completed clearance (K213611) by the FDA this month marks a major milestone for testing fractional exhaled nitric oxide in the US, giving broader access to accurate clinical data for individuals with inflamed airways for patients ages 6 and older, the company says.
The Fenom Pro is a FeNO device used to detect allergic inflammation in asthmatic patients. Its Indication For Use differentiates the device from other options in the market that restrict 6-second test mode to ages 7–10. This new functionality on the Fenom Pro has been available on models outside of the US since 2019.
“The achievement of this indication for use is a giant leap forward in making FeNO testing more comfortable for those patients who find the 10-second test challenging. Accurate data and a shortened test window are a win-win for the patient and clinician who is working to ensure they get the care they need to stay healthy,” said Ryan Leard, CAIRE Diagnostics Chief Operating Officer.
It is estimated that asthma affects more than 300 million individuals worldwide and the Global Initiative for Asthma (GINA) guidelines recommends FeNO measurement as an assessment tool in the management of asthma patients.
FeNO is directly associated with infiltration of eosinophils in the airways and is elevated in individuals with allergic asthma. FeNO can be used in a supportive way to diagnose asthma, to detect nonadherence to inhaled corticosteroids (ICS), and is a great tool for ongoing asthma management.
More information, including updated Fenom Pro Instructions For Use (Software Version 4.2 and later) and exact language on approved indications, is available at Caire’s website.