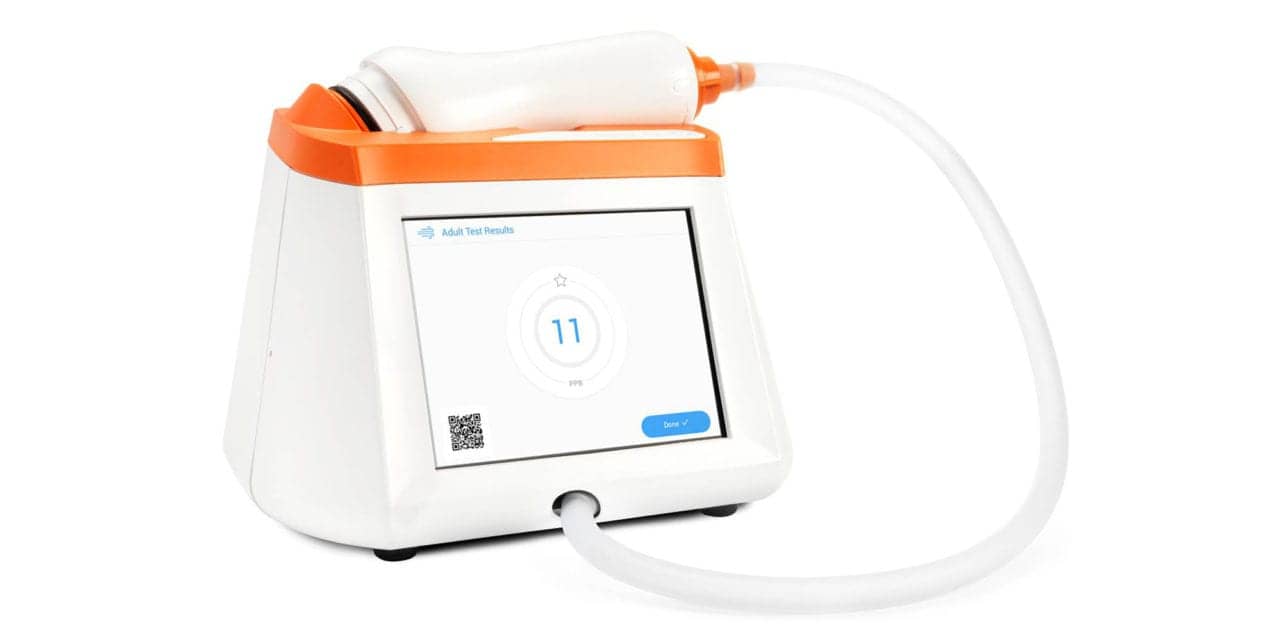
Caire Inc has acquired Spirosure Inc, a California-based developer and manufacturer of the Fenom Pro fractional exhaled nitric oxide (FeNO) device used to detect allergic inflammation in asthmatic patients.
It is estimated that asthma affects more than 300 million individuals worldwide and recent Global Initiative for Asthma (GINA) guidelines recommend FeNO measurement as an assessment tool in the management of asthma patients. FeNO is directly associated with infiltration of eosinophils in the airways and is elevated in individuals with allergic asthma. FeNO can be used to diagnose asthma, to detect nonadherence to inhaled corticosteroids (ICS), to be an early sign of worsening asthmatic inflammation and to manage difficult-to-control asthma because an elevated FeNO level can be predictive of a good response to ICS.
“We are very pleased to have found an excellent strategic buyer in Caire through one of our key investors,” said Solomon Ssenyange, PhD, CEO and Chairman of Spirosure. “Caire shares our commitment to technological leadership and, with its large international organization, is well-positioned to realize the full potential of our proprietary technology both commercially and with regard to our development pipeline. We look forward to an exciting future as part of Caire.”
Spirosure recently launched its first product to market, the Fenom Pro, a reliable and convenient to use device that accurately measures FeNO at parts-per-billion levels using a proprietary sensor technology. The product is intended to be used in the clinic setting and is currently available in the United States, the EU and India.
“We are pleased to add Spirosure’s technology to our market-leading oxygen solution portfolio. FeNO is quickly becoming recognized by the clinical community as an important diagnostic and management tool for clinicians who treat asthma patients,” said Earl Lawson, President and CEO. “The acquisition of Spirosure further diversifies our portfolio and provides access to the 5 billion dollar respiratory diagnostic sector, a market segment where we anticipate rapid growth and adoption.”
Japan-based NGK Spark Plug Co LTD acquired Caire in December 2018 to establish a global foothold in the respiratory therapy business and had previously invested in Spirosure as the latter company’s technology was in development. Spirosure will operate as a division of Caire to be known as Caire Diagnostics Inc, further expanding Caire’s portfolio into the diagnostic segment of respiratory care.