Vivos Therapeutics announced it has received 510(k) clearance from the US Food and Drug Administration (FDA) for treating severe obstructive sleep apnea (OSA) in adults using the Vivos’ removable CARE (Complete Airway Repositioning and/or Expansion) oral appliances.
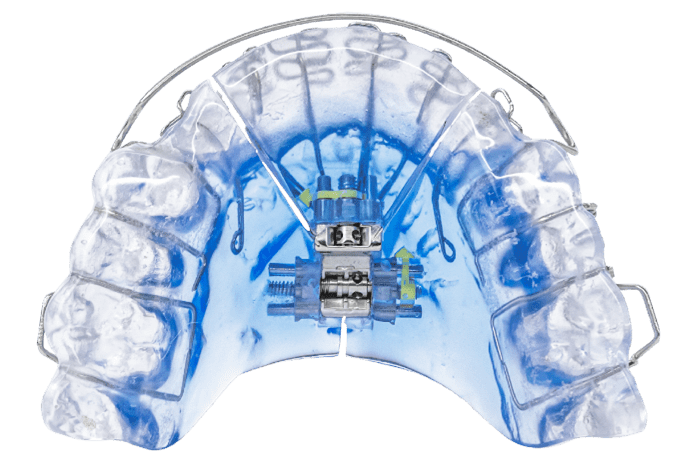
Vivos’ CARE appliances include the flagship DNA oral appliance, the mRNA oral appliance, and the mmRNA oral appliance.
The latest clearance comes 11 months after the FDA granted Vivos 510(k) clearance for the DNA oral appliance to treat mild-to-moderate OSA, and Vivos says it represents the first time the FDA has granted an oral appliance a clearance to treat moderate and severe OSA in adults, 18 years of age and older, along with positive airway pressure and/or myofunctional therapy, as needed.
“Make no mistake: This is a huge development on the landscape of treatments for OSA,” says sleep specialist, author, and lecturer, David McCarty, MD, in a release. “As the medical profession has gained a better understanding of the complex factors contributing to OSA, we now recognize that a critical component to this condition is the form and functionality of the oral vault.”
The statistically significant data submitted to the FDA from 73 severe OSA patients showed that 80% of patients experienced an improvement of at least one classification or at least a 50% improvement in the apnea-hypopnea index, and 97% of patients improved or stayed the same. The average treatment time was 9.7 months.
Treatment results with severe sleep apnea patients were better than with mild and moderate patients. All pre- and post-treatment testing was conducted with no device in the mouth. Vivos’ CARE appliances gradually reposition the hard and soft tissues that define the airway, thereby opening it up and optimizing its function and flow.
In a separate peer-reviewed study, one out of four Vivos patients experienced a complete resolution of their OSA symptoms. No persistent safety issues were found in any patient cohort published or submitted to date, although some patients required aligners following treatment.
Kirk Huntsman, Chairman and CEO of Vivos, says in a release, “This achievement is a pivotal milestone for Vivos and elevates our proven treatment options right into the mainstream of sleep medicine. It is even more important for the millions of severe OSA patients who are desperate for an effective alternative treatment. Before this, severe OSA patients’ only realistic treatment options were CPAP, neurostimulation implants, or other invasive surgeries. Today, they have what we believe is a far more desirable option that is very affordable and doesn’t require surgery or a lifetime of nightly use and intervention.”
Photo caption: Vivos’ DNA oral appliance
Photo credit: Vivos Therapeutics