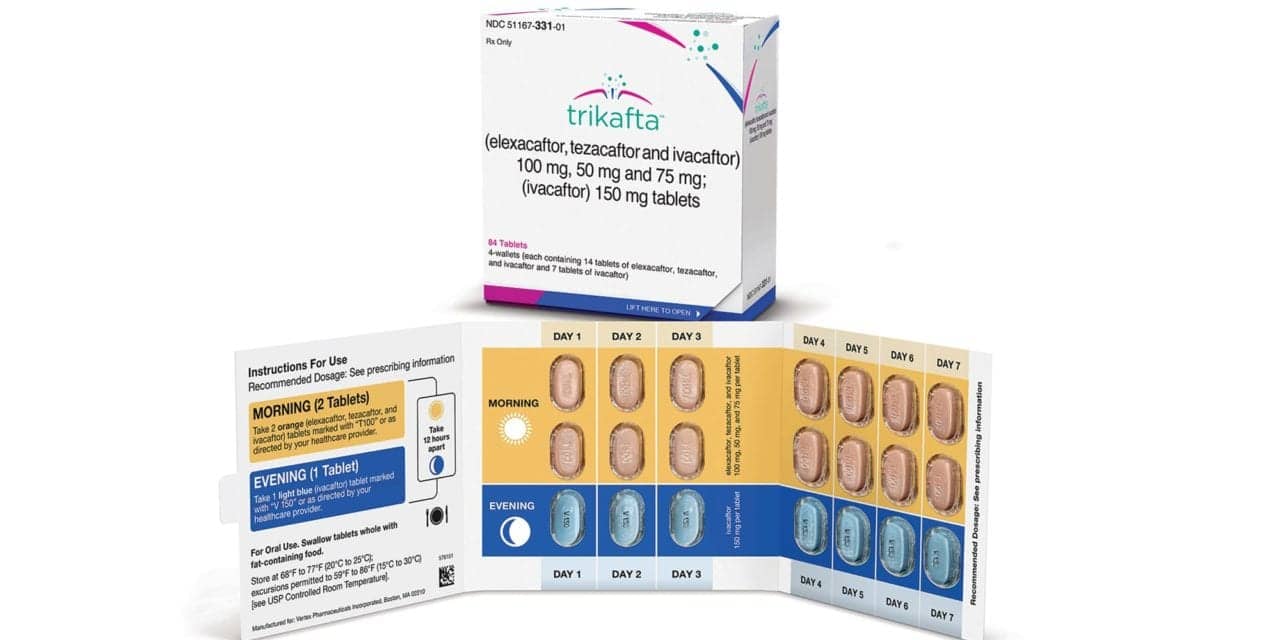
According to a study published in AJRCCM, Vertex Pharma’s Trikafta (elexacaftor/tezacaftor/ivacaftor) is safe and efficacious in children 6 through 11 years of age with at least one F508del-CFTR allele.
The safety and efficacy of Trikafta were evaluated in a Phase 3 study (NCT03691779) involving 66 children age 6 to 11 who have two F508del mutations or one F508del and one minimal function mutation. Children weighing 30 kilograms (about 66 pounds) or more received the full adult daily dose, while children weighing less than 30 kg received half of that dose.
The safety data were consistent with those observed in previous studies with older patients, and the medication was generally well-tolerated.