Pulmatrix Inc will be presenting preclinical data on the pharmacokinetics and potency of PUR1900 during the upcoming 2015 North American Cystic Fibrosis Conference.
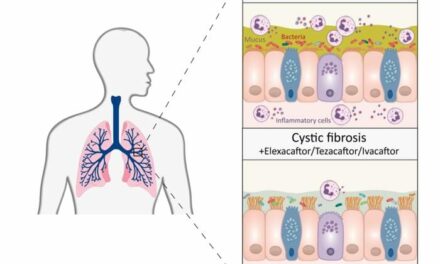
PUR1900 is an iSPERSE (inhaled small particles easily respirable and emitted) formulation incorporating a large, complex anti-fungal compound that can be administered in high therapeutic doses to the lung while minimizing systemic side effects.
It is estimated that nearly 50% of patients with cystic fibrosis (CF) experience pulmonary fungal infections that can cause chronic bronchitis or allergic reactions, resulting in inflammation and poor long term outcomes. PUR1900 is the first inhaled anti-fungal product candidate for CF.
Pulmatrix to Present Preclinical Data on New CF Therapy