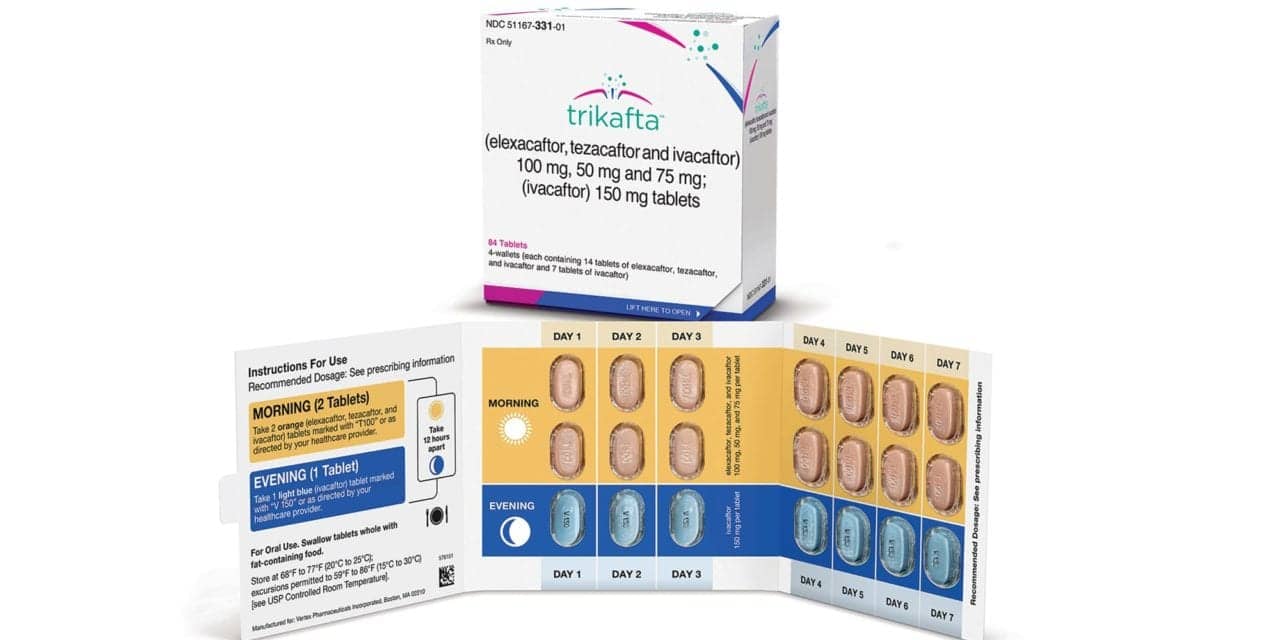
Health Canada granted market authorization for the expanded use of Trikafta (elexacaftor/tezacaftor/ivacaftor and ivacaftor) to include children with cystic fibrosis (CF) ages 2 to 5 years who have at least one copy of the F508del mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene.
Vertex Pharamaceuticals Inc’s Trikafta was previously approved by Health Canada for use in people with CF 6 years and older with at least one F508del mutation.
“With this approval, approximately 330 Canadian children are for the first time eligible for a medicine that treats the underlying cause of their CF,” says Michael Siauw, general manager at Vertex Pharmaceuticals (Canada) Inc, in a release. “We are working closely with private and provincial payers to secure coverage as quickly as possible for this expanded indication of Trikafta.”
This label expansion was supported by a 24-week phase 3 open-label study which enrolled 75 children with CF ages 2 to 5 years old with at least one copy of the F508del mutation to evaluate the safety, pharmacokinetics, and efficacy of Trikafta. The regimen was generally well-tolerated, with a safety profile consistent with that observed in older age groups, and led to improvements in sweat chloride concentration, a measure of CFTR function, and lung function.
“Clinical trials have demonstrated that treatment with Trikafta results in significantly improved health outcomes for many children and adults living with CF, most recently in those as young as 2 years of age,” says Dr Jonathan Rayment, investigator and respirologist at British Columbia Children’s Hospital, clinical assistant professor in the University of British Columbia department of pediatrics, and trial investigator in the Trikafta 2-to-5-year-old clinical trial, in a release. “Hopefully, intervention in the early phases of CF will slow the progression of the disease, allowing these children to live their lives to the fullest.”
In the United States, Trikafta received US Food and Drug Administration approval for this patient population in April.