The etiology, pathology, diagnosis, and treatment of emphysema—a component of COPD—are discussed.
By William V. Wojciechowski, RRT
Pulmonary emphysema is defined, in pathological terms, as the permanent enlargement of air spaces distal to the terminal bronchioles and the destruction of the alveolar walls (without fibrosis). It is a chronic obstructive pulmonary disease (COPD), along with chronic bronchitis. Asthma is excluded from the COPD classification. When the obstructive pattern associated with asthma is reversed, either spontaneously or via medical intervention, the patient is essentially asymptomatic.
Epidemiology and Etiology
As a component of COPD, pulmonary emphysema causes health problems worldwide. Among more than 14 million patients with COPD in the United States, 1.65 million have emphysema and 12.5 million are affected by chronic bronchitis.1 The American Lung Association2 ranks emphysema 15th among chronic conditions contributing to activity limitations, and 44% of patients with emphysema claim to have limitations in their daily living activities resulting from their disease. More than 17,800 deaths are attributable to emphysema in the United States each year.2 The economic impact of emphysema in particular, and of COPD in general, will significantly contribute to the stress imposed on the health care system as average life expectancy increases.
The prevailing cause of pulmonary emphysema is cigarette smoking. Air pollution has been implicated as a cause, but no conclusive data support this notion. Air quality, however, affects individuals who already have emphysema to the extent that air pollution can provoke an exacerbation.
The relationship between cigarette smoking and the incidence of COPD is unusual in that almost 90% of patients with COPD have smoked, but only about 20% of smokers develop COPD.3 Emphysema usually occurs in people 50 or more years old, following decades of smoking. Nonetheless, emphysema develops before 50 years of age in those born with a1–protease-inhibitor deficiency.
Pathogenesis
The pathogenesis of emphysema is grounded in the protease and protease-inhibitor theory. The roots of this theory extend to 1897, when Camus and Gley4 recognized that serum had the capacity to inhibit the proteolytic enzyme trypsin. In 1905, Opie5 discovered that white blood cells contained proteolytic enzymes. Soon after, the suggestion was made that the serum’s inhibitory capacity conferred protection on tissues against proteolytic enzyme destruction. The specific protein that inhibited trypsin was identified later and was called a1-antitrypsin because of its inhibitory activity.6 This protein has been discovered to protect against other proteolytic enzymes and is now often referred to as a1-protease inhibitor (a1-PI).
The normal serum a1-PI concentration is 20 to 50 mol/L or 150 to 350 mg/dL. Serum levels of a1-PI of less than 11 mol/L or 80 mg/dL are considered deficient. Leukocytes, specifically neutrophils (polymorphonuclear leukocytes), contain various proteolytic enzymes in their lysosomes. Alveolar macrophages, derived from monocytes, also contain proteolytic enzymes. The role of these polymorphonuclear leukocytes and alveolar macrophages is to protect the terminal gas-exchange structures of the lungs from inhaled debris and infectious agents. In the process of defending the lungs from these invaders, these phagocytic cells release large quantities of proteolytic enzymes (elastase, cathepsin G, and proteinase) and an array of oxygen radicals (superoxide anions, hydrogen peroxide, hydroxyl radicals, and hypochlorous acid). These proteolytic enzymes and oxygen radicals are microbicidal. The abundance of these microbicidal agents causes the destruction of the cell membrane of the invading microorganism and, ultimately, its demise.
The presence of such toxic products in proximity to the lungs should cause irreversible tissue damage and destruction; however, the lungs normally remain unscathed. The presence of a1-PI and antioxidants in the serum, in cell membranes, and in the alveolar lining fluid layer restrains these toxic products, at the cellular level, from pursuing a potentially lethal course.
The antioxidants located in the lungs are widely distributed and consist of both enzymes and nonenzymes. The major enzymatic antioxidants include superoxide dismutase, glutathione, and catalase. The primary nonenzymatic antioxidants are membrane-bound vitamin C (ascorbic acid) and vitamin E (tocopherol).
Cigarette smoke is a rich source of oxidants. One oxidant, the semiquinone radical, reduces oxygen to superoxide anions. This biochemical event can lead to a cascade of other biochemical reactions. For example, a superoxide anion can react with hydrogen peroxide in the presence of the ferrous ion to produce the hydroxyl radical. The oxidants in cigarette smoke become involved in innumerable biochemical reactions, producing overwhelming numbers of free radicals.
The a1-PI molecule is susceptible to oxidative injury during smoking, rendering a1-PI ineffective in neutralizing proteolytic enzymes. Large numbers of neutrophils are summoned to the site in response to biochemical stimuli to join alveolar macrophages in cleaning up debris from cigarette smoke. In the process, elastase and other proteolytic enzymes are unleashed in the lungs, where these phagocytic cells act virtually unchecked and damage lung tissue. The damage takes the form of degradation of proteoglycans, glycoproteins, elastin, and other extracellular matrix constituents. Elastase also can stimulate inflammation by increasing interleukin-8 synthesis, impair healing by inactivating cytokines and growth factors, and produce pulmonary surfactant abnormalities. These and other injurious events triggered by cigarette smoke damage the lung’s connective-tissue elements and destroy lung parenchyma, thereby producing increased pulmonary compliance, early airway closure during expiration, and air trapping.
Research data challenge the protease and protease-inhibitor theory’s ability to describe the sole or primary pathogenesis for emphysema. Mounting evidence points to collagenase as inflicting damage on fibrillar collagen, which constitutes 50% to 60% of the lung’s extracellular matrix.7 As the major component of the pulmonary extracellular matrix, collagen provides tensile strength in lung tissue and helps to maintain alveolar interdependence.
D’Armiento et al8 demonstrated, in mice, the development of emphysema linked to collagen loss in the absence of a breakdown in elastin. A study9 on the role of collagenase released by alveolar macrophages has revealed the presence of more alveolar macrophages in bronchoalveolar lavage fluid obtained from the lungs of emphysema patients than in fluid from healthy subjects. Increased collagenolytic activity was also found within the lungs of emphysema patients. Recent evidence reveals that the degradation of elastin is not solely responsible for the development of emphysema from cigarette smoking. Collagen breakdown in the lungs appears to play a contributory role.
Alpha-1 Proteinase Inhibitor Deficiency
Alpha-1 proteinase inhibitor deficiency (a1-PI) is a liver-derived glycoprotein consisting of a polypeptide chain of 394 amino acids. Its concentration in the plasma increases as much as fivefold in response to tissue injury and inflammation, affording tissue protection.
a1-PI deficiency is a genetic disorder causing a decreased serum concentration of a1-PI. This autosomal-codominant disorder affects about one in 2,000 live births among people of European descent. Although a number of a1-PI variants exists, most do not cause the serum concentration of a1-PI to drop below 80 mg/dL. The three most common of the 75 known alleles are the M, S, and Z. The M variant is the healthy form that directs the body to produce a normal a1-PI molecule. The S variant is seldom associated with a1-PI deficiency emphysema; however, the Z mutation, characterized by one amino-acid substitution (lysine for glutamine), causes the serum concentration of a1-PI to plummet because this substitution impedes the release of a1-PI from the liver into circulation. Consequently, a person having the Z variant has a higher risk of developing genetic emphysema.
Genetic emphysema accounts for approximately 2% of all emphysema in the United States.10 One current theory regarding a1-PI deficiency is that the low serum level of a1-PI cannot neutralize the proteases released by the alveolar macrophages and neutrophils when these phagocytic cells become active in response to inflammation and pulmonary infections. Therefore, the proteolytic enzymes, over decades, damage lung tissue. The onset of emphysema from a1-PI deficiency often occurs at an earlier age than that caused by cigarette smoking, being seen in patients 30 to 50 years old. Cigarette smoking frequently accelerates the onset of emphysema associated with a1-PI deficiency. Asthma is a common misdiagnosis because the patient generally complains of chronic shortness of breath, chronic cough, wheezing, and pulmonary infections. Making the correct diagnosis of a1-PI deficiency typically takes several visits to multiple physicians over approximately seven years.11
Pathology
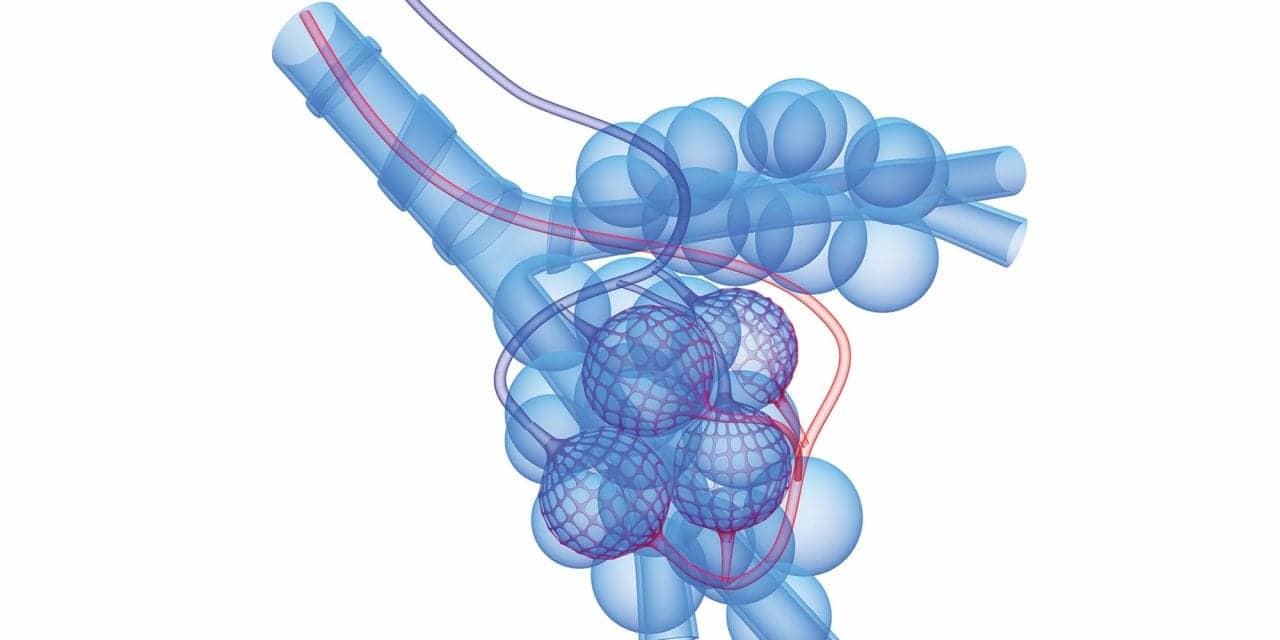
The two basic forms of emphysema are centriacinar (centrilobular) and panacinar (panlobular). An acinus, or lobule, is composed of respiratory bronchioles, alveolar ducts, and alveolar sacs. Centriacinar emphysema involves only the respiratory bronchioles. The alveoli here undergo permanent enlargement and damage to their walls. The alveolar ducts and alveolar sacs distal to the respiratory bronchioles on the same acinus usually do not experience this damage. Centriacinar emphysema is more common than the panacinar form. It predominates in the upper lobes, and is the variety seen among cigarette smokers; however, because the tissue damage during the later stages of centriacinar emphysema often extends to the alveolar ducts and sacs, distinguishing centriacinar from panacinar emphysema at that point becomes impossible.
Panacinar emphysema affects the entire acinus; therefore, the alveoli on the respiratory bronchioles, alveolar ducts, and alveolar sacs become permanently enlarged and experience alveolar-wall destruction. The architecture of the whole acinus is destroyed and replaced by thin-walled air spaces of variable sizes and shapes. These dilated air spaces result from inflammatory destruction of the acinus. This form of emphysema develops frequently in the lower lobes and is the characteristic variety seen in patients with a1-PI deficiency.
Regardless of the form of emphysema present, the structures affected sustain the same architectural rearrangements. Consequently, identical abnormalities prevail in both centriacinar and panacinar emphysema. The damage done to the alveolar walls results in a loss of the lung’s elastic recoil. As a result, effort is needed to perform exhalation. Pursed-lip breathing is generally employed during exhalation to empty the lungs as much as possible.
Enlarged alveoli cause oxygen molecules to travel increased distances to contact alveolar walls to diffuse out of the alveoli and into pulmonary capillaries, many of which have been destroyed. This increases the diffusion time of oxygen across the alveolar-capillary membrane.
Diagnosis
Emphysema often exists in conjunction with chronic bronchitis in the COPD population; nonetheless, emphysema and chronic bronchitis are two distinct diseases. Although a diagnosis of emphysema can, technically speaking, be made only upon postmortem examination, enough evidence can be accumulated from medical history, physical assessment, and diagnostic procedures to make the correct clinical diagnosis.
A patient with emphysema typically seeks medical intervention when shortness of breath occurs while performing ordinary daily activities, such as ascending a flight of stairs. Coughing is usually absent; if it exists, the cough tends to be minor and devoid of sputum. The clinician must obtain thorough social and medical histories because the information gained may reveal that the patient is a cigarette smoker and/or has a relative with a1-PI deficiency.
Characteristic findings can be seen upon physical examination. The patient generally displays tachypnea, lengthened expiratory time, use of accessory muscles of ventilation during inspiration and exhalation, pursed-lip breathing, increased heart rate, and increased anteroposterior chest-wall diameter (barrel chest).
The patient’s posture also may be revealing. Emphysema patients frequently attempt to increase the vertical dimension of the thorax to achieve a mechanical advantage for the muscles of ventilation. For example, when sitting, they place both elbows on the arms of the chair and lean forward. Palpation tends to demonstrate decreased vocal and tactile fremitus because the hyperaerated condition of the lungs creates a suboptimal environment for the transmission of sound waves. Similarly, hyperresonant notes are perceived via percussion, and auscultation reveals diminished or distant breath sounds.
A chest radiograph generally cannot establish the diagnosis of mild emphysema. No remarkable findings appear when mild emphysema exists; however, when emphysema is fully established, classic radiographic findings on an anteroposterior view are typically observed. These findings include bilaterally hyperlucent lungs, flattened hemidiaphragms with widened costophrenic angles, and horizontal ribs. The peripheral vascular markings frequently abate quickly. On the other hand, the markings become prominent when the patient has pulmonary hypertension and cor pulmonale. A lateral view shows an increased retrosternal airspace.
Typically, the heart appears long and narrow because it is influenced by the downward pull created by the flattened hemidiaphragms. In the presence of cor pulmonale, the right ventricle appears enlarged because of right-ventricular hypertrophy.
Spirometry documents the presence of chronic airflow obstruction. Forced vital capacity (FVC) measurement provides data for assessment of expiratory airflow. Measurements of FVC, forced expiratory volume in 1 second (FEV1/FVC), show the presence and degree of airflow obstruction.
A prebronchodilator-postbronchodilator study may indicate some degree of improvement in the quality of the forced expiratory airflow. The FEV1 itself is a useful index of physiological impairment because it correlates closely with the extent of a person’s functional disability and prognosis. An FEV1 of less than 1 L indicates a dismal prognosis.
Carbon monoxide diffusing capacity (dlco) testing is performed in conjunction with spirometry to assist in differentiating emphysema from asthma and chronic bronchitis. In emphysema, the dlco is usually decreased because of the loss of surface area of the alveolar-capillary membrane. The combination of a decreased FVC, a decreased FEV1, a decreased dlco, and increased lung volumes and capacities is generally diagnostic of emphysema.
Arterial blood-gas data tend to vary according to the stage of emphysema. In the mild and moderate stages, the PaO2 and the PaCO2 measurements may remain normal or, while the PaO2 stays normal, the PaCO2 can be decreased (respiratory alkalosis). In the moderately severe and severe forms of emphysema, the patient is likely to be hypoxemic and hypercarbic (respiratory acidosis).
High-resolution CT may be useful in the diagnosis of subclinical or mild emphysema. High-resolution CT scanners furnish images of low-attenuation lesions associated with emphysema.
Treatment
The major goal in treating emphysema is improving the patient’s quality of life. Smoking cessation is a primary focus. Avoidance of exposure to other noxious gases (including secondhand smoke and air contaminants in general) is stressed to lessen the deterioration of lung function.
Many medications are available for emphysema patients. The pharmacological mainstays are bronchodilators and anti-inflammatory agents. The bronchodilators primarily used are b2-agonists and anticholinergics. Two b2-agonists frequently prescribed are albuterol and salmeterol. Ipratropium bromide is an anticholinergic bronchodilator that sometimes affords emphysema patients improved expiratory airflow. A combination of albuterol and ipratropium bromide is also available in a metered-dose inhaler. Not all emphysema patients derive clinical benefit from bronchodilators; however, some clinicians believe that emphysema patients, especially those who have an FEV1 of less than 2 L, should be given a 1-week trial of a bronchodilator.
According to the Global Initiative for Chronic Obstructive Lung Disease (GOLD),12 prolonged treatment with inhaled glucocorticosteroids does not alter long-term deterioration in FEV1 in patients with COPD. Some clinicians prescribe a 2-week trial of an oral glucocorticosteroid to identify patients who respond favorably to these anti-inflammatory agents. These patients are then prescribed an inhaled glucocorticosteroid to minimize the adverse reactions to this drug seen with long-term oral administration. GOLD advocates a trial of 6 weeks to 3 months to identify patients who may experience symptomatic relief and, possibly, benefit from prolonged treatment.
Theophylline has been used; however, its effectiveness in treating emphysema has been questioned because theophylline inhibits multiple phosphodiesterase enzymes. Phosphodiesterase-4 is the primary enzyme in the metabolism of cyclic adenosine monophosphate in smooth muscle. The selective phosphodiesterase-4 inhibitor cilomast, which is a bronchodilator that also reduces inflammation, demonstrates promise in the treatment of emphysema and COPD in general.
Oxygen therapy constitutes the cornerstone of treatment in emphysema. Prolonged use of oxygen for 15 hours per day increases the life expectancies of patients experiencing chronic respiratory failure.13 For patients who have a PaO2 of 55 mm Hg or less (or a pulse-oximetry result of 88% or less), supplemental oxygen is indicated. The administration of oxygen to these patients generally improves gas exchange, decreases the work of the heart, reduces pulmonary vascular resistance, and improves the ability to perform activities of daily living. Oxygen is usually administered via standard nasal cannula or some type of oxygen-conserving device.
Lung-volume–reduction surgery (LVRS) is another method for treating emphysema. In the advanced stages of emphysema, the lungs overfill the thoracic cavity because of the loss of lung elasticity. This condition contributes to airway compression, difficulty in breathing, and the use of accessory ventilatory muscles. LVRS involves reducing the size of the lung by excising a lung section. The smaller lung is better accommodated inside the thorax, and this enables the ventilatory muscles to work more efficiently; however, no randomized controlled studies support the therapeutic benefit of LVRS, compared with nonsurgical intervention.
Single-lung transplantation is performed more commonly among COPD patients than in any other patient population. Its success rate among COPD patients, compared with patients having other diseases, is favorable; however, emphysema patients experience the worst survival rate among patients with chronic airflow limitation. Data14 have shown that patients with the lowest dlco results experience the poorest outcomes.
Replacement or augmentation therapy restores a1-PI serum levels to normal in patients with a1-PI deficiency. Purified human a1-PI is delivered intravenously at a dose of 60 mg/kg every 2 weeks at a yearly cost of approximately $30,000. Attempts are being made to aerosolize a1-PI. Randomized clinical studies investigating the efficacy of intravenous replacement therapy have yet to be conducted.
Gene therapy is being explored. Researchers have used valine to replace methionine at the site where a1-PI is susceptible to oxidative damage. This amino-acid substitution prevents oxidative damage to the a1-PI molecule.
The treatment armamentarium for emphysema is being improved; however, emphysema is a devastating disease that can often be prevented by avoiding cigarette smoking. All healthcare workers should promote smoking prevention and cessation.
RT
William V. Wojciechowski, RRT, is associate professor and chair, baccalaureate degree respiratory therapy program, Department of Cardiorespiratory Care, University of South Alabama, Mobile.
References
1. Flaherty KR, Kazerooni EA, Martinez FJ. Differential diagnosis of chronic airflow obstruction [review article]. J Asthma. 2000;37:201-223.
2. American Lung Association. Data and statistics. Available at: http://www.lungusa.org/. Accessed November 2002.
3. Repine JE, Bast A, Lankhorst I, The Oxidative Study Group. Oxidative stress in chronic obstructive pulmonary disease. Am J Crit Care Med. 1997;156:341-357.
4. Camus L, Gley E. Action du serum sanguin sur quelques ferments digestifs. Comptes Rendus de Société Biologique. 1897;49:825.
5. Opie EL. Enzymes and anti-enzymes of inflammatory exudates. J Exp Med. 1905;7:316-334.
6. Schultz HE, Heide K, Haupt H. Alpha1-antitrypsin aus human-serum. Wien Klin Wochenschr. 1962;40:420-427.
7. Lang MR, Fiaux GW, Stewart JA, Hulmes DJ, Lamb D. Collagen content of alveolar wall tissue in emphysematous and non-emphysematous lungs. Thorax. 1994;49:319-326.
8. D’Armiento J, Dalala SS, Okada Y, Berg RA, Chada K. Collagenase expression in the lungs of transgenic mice causes pulmonary emphysema. Cell. 1992;71:955-961.
9. Imai K, Dalala S, Chen E, et al. Human collagenase (matrix metalloproteinase-1) expression in the lungs of patients with emphysema. Am J Respir Crit Care Med. 2001;163:786-791.
10. Köhnlein T, Klein H, Welte T. Alpha-1-proteinaseninhibitor-mangel. diagnostik, krankheitsverlauf und therapieoptionen. Med Klin. 1999;94:371-376.
11. Alpha-1 Association. Search alpha-1. Available at: http://www.alpha1.org/. Accessed November 2002.
12. Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Diseases (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163:1256-1276.
13. Nocturnal Oxygen Therapy Trial Group. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Ann Intern Med. 1980;3:391-398.