The US FDA has approved the Zephyr Endobronchial Valve System for treating severe emphysema patients, according to manufacturer Pulmonx Corp.
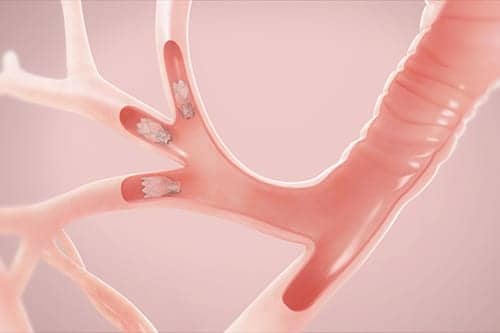
The Pulmonx Zephyr Endobronchial Valves are implantable bronchial valves indicated for the bronchoscopic treatment of adult patients with hyperinflation associated with severe emphysema in regions of the lung that have little to no collateral ventilation.
Zephyr is the first minimally-invasive device approved in the United States for treating patients with severe emphysema, a progressive and life-threatening form of COPD.
The approval is based on positive clinical data from the pivotal LIBERATE Study and two other multicenter randomized control trials. In the LIBERATE study, patients treated with Zephyr Valves were able to breathe easier, be more active and energetic, be less short of breath, and enjoy a significantly improved quality of life compared to patients who received medical management alone.
As stated in the Summary of Safety and Effectiveness Data, the FDA granted the Zephyr Valve an expedited review because it “represents a breakthrough technology as the device offers bronchoscopic lung volume reduction without surgery and its associated risks. This device offers significant clinically meaningful advantage over the current standard of care and, therefore, its availability is also in the best interest of patients.”
“Zephyr Valves are a major step forward in treating severe emphysema patients who consistently feel short of breath despite all the medications we can offer. I have seen Zephyr Valve-treated patients getting back to a more active life doing the things they enjoy. As a physician, it is very gratifying to have a new treatment that can restore a patient’s confidence and change their life for the long term,” said Gerard Criner, MD, FACP, FACCP, Chair and Professor of Thoracic Medicine and Surgery, Lewis Katz School of Medicine at Temple University, and lead investigator for the LIBERATE Study.
More than 15 million Americans suffer from COPD, and 3.5 million of those patients have emphysema. Despite using COPD medications, over one million emphysema patients continue to suffer symptoms of hyperinflation, in which air becomes trapped in the lungs and prevents new air from coming in, causing severe shortness of breath. The inability to get enough air often prevents these patients from doing simple daily activities, such as bathing, getting dressed, performing household chores and walking, without pausing to catch their breath. Until now, the only other options for these patients were highly invasive treatments such as lung volume reduction surgery or lung transplantation.
During a bronchoscopic procedure requiring no cutting or incisions, tiny Zephyr Valves are placed in the airways to occlude a diseased part of the lungs and reduce hyperinflation. This helps the healthier parts of the lungs to expand and lifts pressure off the diaphragm, thereby decreasing shortness of breath and making breathing easier.
Patients most likely to benefit from Zephyr Valve treatment can be identified with assessment tools also offered by Pulmonx. Physicians use the Pulmonx Chartis Pulmonary Assessment System and StratX Lung Analysis Platform to help identify potential responders to Zephyr Valve treatment.
Since 2007, more than 14,000 patients have been treated with the Zephyr Valve worldwide. Zephyr Valve treatment is included in emphysema treatment guidance issued by leading health organizations worldwide, including the Global Initiative for Chronic Obstructive Lung Disease (GOLD) and the UK’s National Institute for Health and Care Excellence (NICE).









How do I qualify for this procedure and where can I get it done? Is the VA doing this procedure?
Hey Roger,
Temple University Hospital in Philadelphia will be the main hospital to preform the procedure. Here is the link to the the lung center. https://lung.templehealth.org/
They will be able to answer any further questions.
Thanks,
Matthew Basile RCP
If this is available in the USA, where is it being offered and performed?
Hey Linda,
The main center in the USA is Temple University Hospital. Here is the link for further information, https://lung.templehealth.org/
Thanks,
Matthew Basile RCP
I wasn’t approved for LVRS, maybe this will be for me and a new life.
I was tested for this during clinical trials and did not qualify because my lungs weren’t in sync with one another (or something like that). Would this still prevent me from receiving these valves? Also, what is the age limitation for this procedure? I turned 78 in May. I await your response. Thank you.
Hey lorri,
The original age cut off for the trial was 75. For the procedure, up to 80 will be considered. Temple University Hospital in Philadelphia will be the main center for the procedure. Here is the link for are lung center. They can answer all your questions. https://lung.templehealth.org/
Thanks,
Matthew Basile RCP
What is the age limitation on this procedure?
I would like to know how I can find out who does the procedures I live in South Florida and I would love to learn more as I have COPD very severe and would love to see if this procedure would help me any information would be appreciated.
Patrick J Migliore
954-818-2085
Hey Patrick,
Temple University Hospital in Philadelphia will be the main center to perform the EBV procedures. Here is the link to temples lung center and they can answer any further questions. https://lung.templehealth.org/
Thanks,
Matthew Basile RCP
I would check into Shands Hospital (Gainesville, Florida)and UAB hospital (Birmingham, Alabama). They are both large teaching hospitals and are much closer to you.
I am currently an RT student and would like more information on the Zephyr. I am doing a case study on a new device and would like to know more about this device; cost of the device, who it is being used on, will the defected lung lobes eventually work again, how long the device needs to be kept in a patient, etc. Thank you!
I would also like more information I’m a RT doing a oral presentation over this