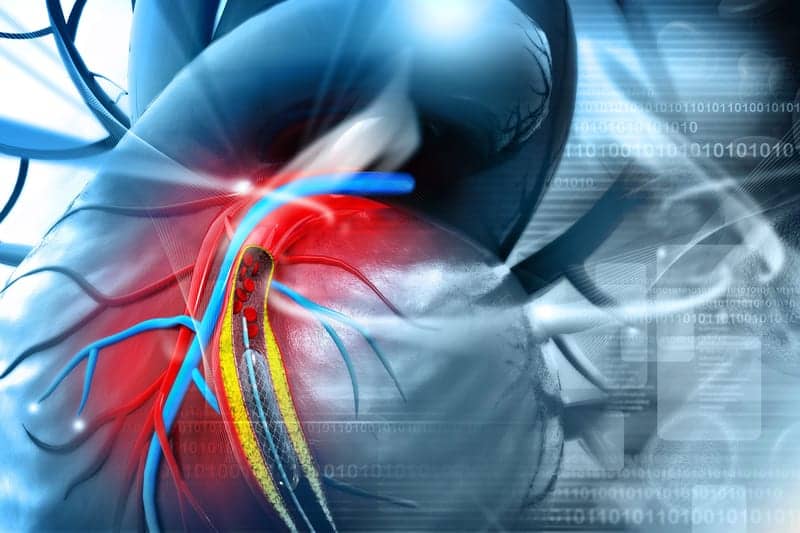
The US Food and Drug Administration (FDA) granted premarket approval for the Cordis Palmaz Mullins XD pulmonary stent for pediatric patients receiving treatment for pulmonary artery stenosis.
Pulmonary artery stenosis is a narrowing of a pulmonary artery, which is a serious form of congenital heart disease. The stent is placed in the pulmonary artery through a catheter inserted through the patient’s blood vessel. The stent addresses the critical narrowing of the pulmonary artery and keeps the pulmonary artery open, decreasing the pressure load on the right ventricle of the heart while improving blood flow to the lungs.
This approval marks the first device of its kind approved to treat pulmonary artery stenosis in pediatric patients that offers an additional treatment option for patients, according to a notice from the FDA. “This device approval is supported by real-world evidence obtained from registry data,” says Bram Zuckerman, MD, director of the office of cardiovascular devices in the FDA’s Center for Devices and Radiological Health, in a release. “Real-world evidence can help overcome some of the challenges presented when studying medical devices in pediatric populations. This approval is a great example of the use of real-world evidence to advance the care of pediatric patients.”
According to the premarket approval, the Palmaz Mullins XD pulmonary stent is indicated for the non-emergency treatment of pulmonary artery stenosis in pediatric patients who weigh at least 10 kg with two ventricle anatomy.