By John D. Davies, MA, RRT, FAARC
Mechanical ventilation (MV) is a life-saving intervention in patients with respiratory failure and other disease entities. However, prolonged mechanical ventilation is associated with a number of complications including but not limited to: ventilator-induced lung injury (VILI), ventilator-associated pneumonia (VAP), increased ICU and hospital length of stay, and increased financial burden. The challenge for bedside clinicians is the ability to optimize the beneficial effects of mechanical ventilation while, at the same time, identifying the appropriate time to liberate their patients from the ventilator.
Historically, weaning from MV has been carried out by a series of gradual reductions in ventilator support. The patient, in turn, would gradually assume more of the work of breathing (WOB), thus “training” the patient’s respiratory musculature in preparation to breathe without the endotracheal tube (ETT). This was generally accomplished using synchronized intermittent mandatory ventilation (SIMV) or pressure support ventilation (PS). Once a “minimal setting” was reached, the clinical team would decide whether extubation was indicated.
In 2001, the process of weaning from MV came under intense scrutiny as a task force consisting of experts from the American College of Chest Physicians (ACCP), the Society of Critical Care Medicine (SCCM), and the American Association for Respiratory Care (AARC) published a series of evidence-based recommendations for weaning and discontinuation of ventilator support.1 The first eight recommendations address the earlier, more acute weaning phases while the last four address issues with prolonged mechanical ventilation. The goal of this article is to discuss the first eight recommendations and examine evidence-based literature relating to them.
Recommendation #1: In patients requiring mechanical ventilation for >24 hours, a search for all the causes that may be contributing to ventilator dependence should be undertaken. This is particularly true in the patient who has failed attempts at withdrawing the mechanical ventilator. Reversing all possible ventilatory and nonventilatory issues should be an integral part of the ventilator discontinuation process.1
Causes of ventilator dependence can be either disease-related or clinician-related. Disease-related include processes that increase the mechanical load on the respiratory system. Factors like decreases in compliance, increases in resistance, presence of intrinsic positive end-expiratory pressure (PEEP), and ventilation/perfusion mismatching can all lead to an increased load on the respiratory musculature. Cardiac tolerance of this increased load can become an issue in patients with cardiovascular compromise. Ventilatory, nutritional, and pharmacologic support can help alleviate some of the disease-imposed load. Clinicians also can unwittingly contribute to an increased load for the patient if they don’t properly match mechanical ventilation support with patient demand. Trigger, flow, and/or cycle asynchrony can cause the patient to exert unwanted work fighting with the ventilator to get what they want. The prevention of patient-ventilator asynchrony depends on the clinicians’ ability to recognize both that it is happening and what to do to correct it. The newer generation ventilators have much improved graphics, which allow for more accurate graphic analysis, and they are much more sensitive to patients’ requirements. So, the key is to identify reversible reasons for ventilator dependence and correct them—both disease and clinician-related.
Recommendation #2: Patients receiving mechanical ventilation for respiratory failure should undergo a formal assessment for discontinuation potential if the following criteria are satisfied: 1) Evidence for some reversal of the underlying cause for respiratory failure; 2) Adequate oxygenation (eg, Pao2/Fio2 ratio >150 to 200, requiring positive end-expiratory pressure [PEEP] less than 5-8 cm H2O; Fio2 less than 0.4 to 0.5) and pH (eg, >7.25); 3) Hemodynamic stability, as defined by the absence of active myocardial ischemia and the absence of clinically significant hypotension (ie, a condition requiring no vasopressor therapy or therapy with only low-dose vasopressors such as dopamine or dobutamine, less than 5 µg/kg/min); and 4) The capability to initiate an inspiratory effort.
This is often termed a “wean screen”—the search for objective indices indicating the readiness to wean. Prudent subjective clinical assessment, however, cannot be ignored either. For readiness to wean, the clinician must ask questions such as: How does the respiratory pattern look? Does the patient appear to be doing too much work? Is the patient agitated? Is the patient using accessory muscles? Is the breathing pattern regular?
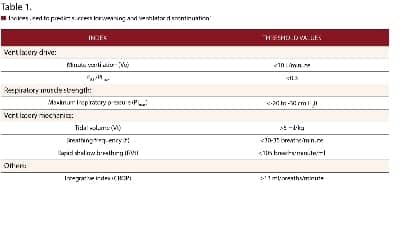
These are but a few of the questions that make up a proper assessment. Because the identification of the right time to “wean” is based on many subjective factors and is often inaccurate, attempts have been made to substitute objective criteria (weaning predictors) to guide the decision-making process. Some criteria that have been used include minute ventilation (Ve), respiratory rate (RR or f), tidal volume (Vt), maximum inspiratory pressure (PImax), ratio of airway occlusion pressure 0.1 second after the onset of inspiratory effort to maximum inspiratory effort (P0.1/PImax), rapid shallow breathing index (expressed as RSBI or f/Vt), and the CROP (Compliance, RR, Oxygenation, and Pressure) index. Threshold values for these indices are listed in Table 1.2
The RSBI has received the most attention as a potential weaning predictor. An RSBI value of An RSBI value of less than 105 is considered “weanable.” The RSBI held promise when initially studied.3 However, the RSBI has more predictive value when used in conjunction with the other indices. The CROP index was an effort to merge a number of indices into one predictor. The formula is: CROP (ml/breath/min) = [Cdyn x MIP x (Pao2/PAo2)]/RR where Cdyn = dynamic compliance and and MIP = airway pressure.3 The CROP index is cumbersome to use and, unfortunately, has a poor predictive power as well for assessing weaning readiness.
All of these above-mentioned criteria can help guide decision making, but, strictly on their own merit, they are poor predictors of weaning capability.4 Other wean screen variables that have been cited are intact airway defenses and normothermia.2 Another factor to consider is the level of sedation. Kress et al showed that daily interruptions of patients receiving mechanical ventilation reduced the duration of ventilation from 7.3 days to 4.9 days (p=.004).5
Recommendation #3: Formal discontinuation assessments for patients receiving mechanical ventilation for respiratory failure should be performed during spontaneous breathing rather than while the patient is still receiving substantial ventilatory support. An initial brief period of spontaneous breathing can be used to assess the capability of continuing on to a formal spontaneous breathing trial (SBT). The criteria with which to assess patient tolerance during SBTs are the respiratory pattern, the adequacy of gas exchange, hemodynamic stability, and subject comfort. The tolerance of SBTs lasting 30 to 120 minutes should prompt consideration for permanent ventilator discontinuation.1
Essentially, once the wean screen has been passed, how does the clinician now assess for extubation potential? The recommendation from the task force is that the formal discontinuation assessment be performed during a trial SBT as opposed to “substantial” ventilator support. The SBT should last 30 to 120 minutes. The criteria to assess patient tolerance include the respiratory pattern, adequacy of gas exchange, hemodynamic stability, and subjective comfort. The SBT was shown to be superior to gradual reductions in both SIMV and pressure support ventilation.6
The use of an SBT as the formal discontinuation assessment also has been recommended by a more recent task force. This task force consisted of experts from the European Respiratory Society, the American Thoracic Society, the European Society of Intensive Care Medicine, the SCCM, and the Société de Réanimation de Langue Française.7 The objective indices discussed in the previous paragraph can be applied here as well for assessing tolerance, but the same caveats in terms of predictive power also apply. Again, however, the indices help support the subjective assessment but do not fare well as independent predictors. The RSBI is commonly used as a surrogate predictor for SBT assessment. However, two recent studies looked at the extubation predictive value of the RSBI and found that the RSBI could not predict extubation failure.8,9
A new knowledge-based automated weaning system recently has been introduced. SmartCare/PS (Dräger Medical, Lübeck, Germany) is designed to continuously monitor the patient’s respiratory status and automatically adjust the PS level. There was some hope that this mode could hasten mechanical ventilation discontinuation. However, studies to this point have not shown this to be the case.10,11 It still may be of use in clinical settings, however, where an institution may not have sufficient clinical staff to perform SBTs in a timely manner.
There are several purported ways to physically perform the SBT. The three most common methods are the T-tube trial (the patient is taken off the ventilator and placed on oxygen in a flow-by manner), low levels of continuous positive airway pressure (CPAP), and the use of low levels of PS (5-7 cm H2O) with CPAP. The use of PS is thought to overcome the resistance of the ETT to reduce the work of breathing. Some contend, however, that the T-tube trial is a more accurate indication of how the patient will do once the ETT is removed due to the presence of inflamed natural upper airways.12 There is also no consensus on whether to use an elevated baseline pressure (CPAP in the T-tube trial or PEEP with PS). The exception to this is a patient who has intrinsic PEEP. Applying an elevated baseline will help these patients with triggering or getting a breath and thereby decrease the work of breathing. Reisssmann et al found that patients with COPD were more likely to pass an SBT with 5-7 cm H2O of CPAP.13
To this point, however, none of the three methods seems to be superior and both of the previously mentioned task forces recommend that either of the methods can be used to perform the SBT.1,7
Automatic tube compensation (ATC) is a relatively new ventilator mode designed to compensate for the nonlinear pressure drop across the ETT during spontaneous breathing. It has been suggested that this may be a more effective way to administer the SBT. However, the literature to this point shows that ATC appears to be only just as effective as PS.14-17
Recommendation #4: The removal of the artificial airway from a patient who has successfully been discontinued from ventilatory support should be based on assessments of airway patency and the ability of the patient to protect the airway.1
Essentially, once the patient has been cleared from a respiratory and hemodynamic perspective, the decision to actually extubate should hinge on the amount of secretions and the ability to clear those secretions.
Mental status has been a point of contention as well—does the patient have to be awake and alert to be considered for extubation? The literature is conflicting in terms of whether mental status is an independent risk factor for extubation success. Studies by Mokhlesi et al and Namen et al suggested that extubation success was associated with a higher Glasgow Coma Scale (GCS) rating.18,19 Salam and colleagues studied the ability of patients to follow four specific commands (open eyes, follow with their eyes, stick out their tongue, and grasp a hand) and found that patients who could not follow the commands were more likely to require reintubation.20 However, in these studies, the inability to cough and presence of secretions were either separate independent risk factors or they weren’t factored in at all.
There are also some studies that show that patient populations unable to follow commands are not at an elevated risk of extubation failure. Frutos-Vivar and Beuret both found that patients with a poor ability to cooperate were at no more risk of extubation failure than patients who could cooperate.21,22 Also, it is difficult to define mental status in a standardized way. The result is that different studies define it in different ways.
Ultimately, it is difficult to say that mental status alone was the reason for extubation failure. A large prospective randomized control trial that can standardize the definition of mental status and account for cough ability and secretion load would certainly help clarify some of these issues.
Recommendation #5: Patients receiving mechanical ventilation for respiratory failure who fail an SBT should have the cause for the failed SBT determined. Once reversible causes for failure are corrected, and if the patient still meets the SBT criteria, subsequent SBTs should be performed every 24 hours.1
There is very little literature supporting a gradual wean down to minimal support before the SBT is attempted. Gradual weans come with the very real risk of overloading the respiratory muscles, possibly hindering future SBTs. Also, the gradual wean may increase the time on the ventilator if the patient’s ability to perform an SBT is not recognized.
Recommendation #6: Patients receiving mechanical ventilation for respiratory failure who fail an SBT should receive a stable, nonfatiguing, comfortable form of ventilatory support.1
The use of a comfortable, nonfatiguing ventilatory mode also is supported in the European Task Force Consensus.7 The mode chosen should ultimately be the one that best achieves optimal patient-ventilator synchrony. Attention should be centered on optimizing trigger, flow, and cycle synchrony between the patient and the ventilator.
Recommendation #7: Anesthesia/sedation strategies and ventilator management aimed at early extubation should be used in postsurgical patients.1
I believe that this recommendation should not apply solely to the postsurgical patients. As mentioned earlier, Kress and colleagues initially showed that daily interruption of sedation decreases duration of mechanical ventilation and ICU length of stay.5 These results were supported in a larger, more recent study. Girard et al showed that a spontaneous awakening trial (SAT) followed by an SBT reduced duration of mechanical ventilation and ICU length of stay.23
Recommendation #8: Weaning/discontinuation protocols that are designed for nonphysician health care professionals (HCPs) should be developed and implemented by ICUs. Protocols aimed at optimizing sedation also should be developed and implemented.1
The study by Girard mentioned above not only showed that SATs, when combined with SBTs, can shorten the duration of MV.23 He also used protocols in this study to further optimize the weaning process.23 Weaning protocols are perfect for segueing into incorporation of SATs and SBTs in that they provide a standardized approach. Appropriately designed protocols offer advantages such as standardization of practice, improvements in multidisciplinary communication, and overall streamlining of the process. A recent meta-analysis examined a number of studies relating to protocol-driven weaning and concluded that the use of a weaning protocol can result in decreased duration of mechanical ventilation, weaning duration, and ICU length of stay.24
In summary, the recommendations from a task force made up of experts from the ACCP, SCCM, and AARC have, for the most part, stood the test of time. Most of the recommendations still apply today. SBTs should be done as early as possible following a wean screen. If the SBT is passed, then an adequate cough and manageable secretion amount should be present before extubation is carried out. If the SBT is not passed, then the patient should be placed on a comfortable, synchronous mode of ventilation that should be used until the next SBT is performed.
All the while, reversible causes of weaning delay should be investigated. The use of SATs coupled with SBTs and weaning protocols also serves to minimize the duration of mechanical ventilation. Newer modes of MV are emerging that may have the potential to help speed up the ventilator discontinuation process. However, randomized controlled trials are needed to assess whether that potential can be translated into clinical success.
For the time being, these recommendations should be adhered to, to optimize the ventilator liberation process. ?
John D. Davies, MA, RRT, FAARC, is clinical research coordinator in Adult Critical Care at Duke University Medical Center, Durham, NC. For further information, contact [email protected].
1. MacIntyre NR, Cook DJ, Ely EW, et al. Evidence-based guidelines for weaning and discontinuing ventilator support: a collaborative task force facilitated by the American College of Chest Physicians, the American Association for Respiratory Care, and the American College of Critical Care Medicine. Chest. 2001;120(suppl):375S-395S.
2. El-Khatib MF, Bou-Khalil P. Clinical review: liberation from mechanical ventilation. Crit Care. 2008;12:221.
3. Yang KL, Tobin MJ. A prospective study of indexes predicting the outcome of trials of weaning from mechanical ventilation. N Engl J Med. 1991;324:1445-1450.
4. Conti G, Montini L, Pennisi MA, et al. A prospective, blinded evaluation of indexes proposed to predict weaning from mechanical ventilation. Intensive Care Med. 2004;30:830-836.
5. Kress JP, Pohlman AS, O’Connor MF, et al. Daily interruptions of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342:1471-1477.
6. Esteban A, Frutos F, Tobin MJ, et al. A comparison of four methods of weaning patients from mechanical ventilation: the Spanish Lung Failure Collaborative Group. N Engl J Med. 1995;332:345-350.
7. Boles JM, Bion J, Connors A, et al. Weaning from mechanical ventilation. Eur Respir J. 2007;29:1033-1056.
8. Teixeira C, Teixeira PJ, Hoher JA, de Leon PP, Brodt SF, da Siva Moreira J. Serial measurements of f/Vt can predict extubation failure in patients with f/Vt < or = 105? J Crit Care. 2008;23:572-6.
9. Tanios MA, Nevins ML, Hendra KP, et al. A randomized, controlled trial of the role of weaning predictors in clinical decision making. Crit Care Med. 2006;34:2530-2535.
10. Rose L, Presneill JJ, Johnston L, et al. A randomized, controlled trial of conventional versus automated weaning from mechanical ventilation using SmartCare TM/PS. Intensive Care Med. 2008;34:1788-1795.
11. Schädler D, Engel C, Elke G, et al. Automatic control of pressure support for ventilator weaning in surgical intensive care patients. Am J Respir Crit Care Med. 2012;185:637-644.
12. Mehta S, Nelson DL, Klinger JR, et al. Prediction of post-extubation work of breathing. Crit Care Med. 2000;28:1341-1346.
13. Reissmann HK, Ranieri VM, Goldberg P, et al. Continuous positive airway pressure facilitates spontaneous breathing in weaning chronic obstructive pulmonary disease patients by improving breathing pattern and gas exchange. Intensive Care Med. 2000;26:1764-1772.
14. Haberthur C, Mols G, Elsasser S, et al. Extubation after breathing trials with automatic tube compensation, T-tube, or pressure support ventilation. Acta Anaesthesiol Scand. 2002;46:973-979.
15. Figueroa-Casas JB, Montoya R, Arzabala A, et al. Comparison between automatic tube compensation and continuous positive airway pressure during spontaneous breathing trials. Respir Care. 2010;55:549-554.
16. Cohen JD, Shapiro M, Grozovski E, et al. Extubation outcome following a spontaneous breathing trial with automatic tube compensation versus continuous positive airway pressure. Crit Care Med. 2006;34:682-686.
17. Cohen J, Shapiro M, Grozovski E, et al. Prediction of extubation outcome: a randomized, controlled trial with automatic tube compensation vs. pressure support ventilation. Crit Care. 2009;13:R21.
18. Mokhlesi B, Tulaimat A, Gluckman TJ, et al. Predicting extubation failure after successful completion of a spontaneous breathing trial. Respir Care. 2007;52:1710-1717.
19. Namen AM, Ely EW, Tatter SB, et al. Predictors of successful extubation in neurosurgical patients. Am J Respir Crit Care Med. 2001;163:658-664.
20. Salam A, Tilluckdharry L, Amoateng-Adjepong Y, Manthous CA. Neurologic status, cough, secretions and extubation outcomes. Intensive Care Med. 2004;30:1334-1339.
21. Frutos-Vivar F, Ferguson ND, Esteban A, et al. Risk factors for extubation failure in patients following a successful spontaneous breathing trial. Chest. 2006;130:1664-1671.
22. Beuret P, Roux C, Auclair A, et al. Interest of an objective evaluation of cough during weaning from mechanical ventilation. Intensive Care Med. 2009;35:1090-1093.
23. Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial)): a randomized controlled trial. Lancet. 2008;371:126-134.
24. Blackwook B, Alderdice F, Burns K, et al. Use of weaning protocols for reducing duration of mechanical ventilation in critically ill adult patients: Cochrane systematic review and meta-analysis. BMJ. 2011;342:c7237.